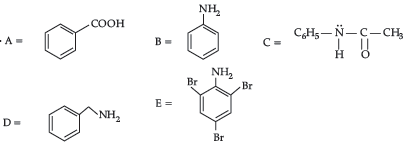
Please refer to Class 12 Chemistry Sample Paper With Solutions Set C provided below. The Sample Papers for Class 12 Chemistry have been prepared based on the latest pattern issued by CBSE. Students should practice these guess papers for class 12 Chemistry to gain more practice and get better marks in examinations. The Sample Papers for Chemistry Standard 12 will help you to understand the type of questions which can be asked in upcoming examinations.
Sample Paper for Class 12 Chemistry With Solutions Set C
Topic-1
Aldehydes and Ketones
Very Short Answer-Objective Type Questions
A. Multiple choice Questions:
Question. The reagent which does not react with both, acetone and benzaldehyde.
(a) Sodium hydrogen sulphite
(b) Phenyl hydrazine
(c) Fehlings’ solution
(d) Grignard reagent
Answer
C
Question. Cannizzaro reaction is not given by____________

Answer
A
Question. Compounds A and C in the following reaction are :
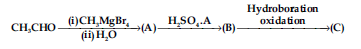
(a) identical
(b) positional isomers
(c) functional isomers
(d) optical isomers
Answer
B
Question. Which of the following compounds will give butanone on oxidation with alkaline KMnO4 solution?
(a) Butan-1-ol
(b) Butan-2-ol
(c) Both of these
(d) None of these
Answer
B
Question. In Clemmensen reduction carbonyl compound is treated with
(a) zinc amalgam + HCl
(b) sodium amalgam + HCl
(c) zinc amalgam + nitric acid
(d) sodium amalgam + HNO3
Answer
A
B. Match the following :
Question. Match the species given in Column I with those mentioned in Column II.
Column Column II
(i) Cinnamaldehyde (a) Pentanal
(ii) Acetophenone (b) Prop-2-enal
(iii) Valeraldehyde (c) 4-Methylpent-3-en- 2-one
(iv) Acrolein (d) 3-Phenylprop- 2-enal
(v) Mesityl oxide (e) 1-Phenylethanone
Answer. (i) → (d)
(ii) → (e)
(iii) → (a)
(iv) → (b)
(v) → (c)
C. Answer the following :
Question. Write the IUPAC name of the following :
CH3 – CH2 – CHO
Answer. Propanal.
Question. Write the IUPAC name of
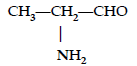
Answer. 2-Aminopropanal.
Question. Write the IUPAC name of :

Answer. Pent-2-en-1-al.
Question. Draw the structure of 3-methylpentanal.
Answer.
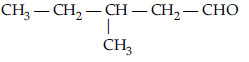
Question. Write the structure of 3-methylbutanal.
Answer.
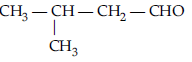
Question. Write the structure of 4-chloropentan-2-one.
Answer.
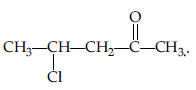
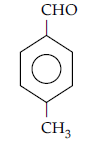
Question. Write the structure of p-methyl benzaldehyde molecule.
Answer.
Question. Draw the structure of the compound named 4-methyl pent-3-en-2-one
Answer.
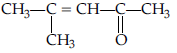
Question. What type of aldehydes undergo cannizaro reaction?
Answer. Having no a-hydrogen
Question. Arrange the following compounds in an increasing order of their reactivity in nucleophilic addition reactions : ethanal, propanal, propanone, butanone.
Answer. Increasing order of reactivity :
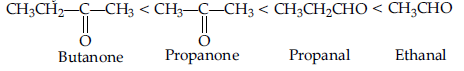
Question. An aromatic organic compound ‘A’ with molecular formula C8H8O gives positive DNP and iodoform tests. It neither reduces Tollens’ reagent nor does it decolourise bromine water. Write the structure of ‘A’.
Answer. C6H5COCH3
Question. (CH3)3C-CHO does not undergo aldol condensation. Comment
Answer. No a-H is present
Question. Out of CH3CH2COCH2CH3 and CH3CH2CH2 COCH3 which gives iodoform test.
Answer. CH3CH2CH2COCH3 will give iodoform test as it has a terminal Ketomethyl group.
Question. Give the chemical test to distinguish between the following pairs of compounds.
(i) Propanal and propanone.
(ii) Benzaldehyde and Benzoic acid.
Answer. (i) Propanal gives silver mirror on reaction with
Tollen’s reagent while propanone does not give.
(ii) Benzoic acid evolves CO2 gas with NaHCO3 while benzaldehyde does not evolve CO2.
Short Answer Type Questions
Question. Name the reagents used in the following reactions :
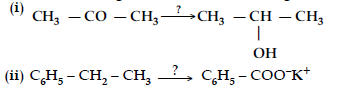
Answer. (i) LiAlH4/ NaBH4 /H2, Pt
(ii) KMnO4, KOH
Question. Write the reagents required in the following reactions :
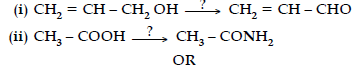
Arrange the following compounds in increasing order of their property as indicated :
(i) CH3COCH3, C6H5COCH3, CH3CHO (reactivity towards nucleophilic addition reaction)
(ii) Cl – CH2 – COOH, F – CH2 – COOH, CH3 – COOH
(acidic character)
Answer. (i) PCC / Cu at 573 K.
(ii) NH3, D(heat).
OR
(i) C6H5COCH3<CH3COCH3<CH3CHO.
(ii) CH3COOH<Cl – CH2 – COOH<F – CH2 – COOH
Question. Complete the following reactions :
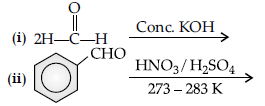
Answer.
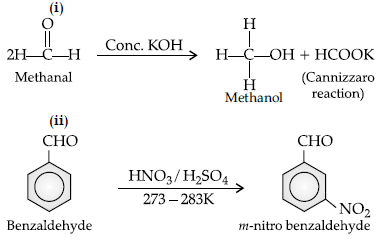
Question. Write the equations involved in the following reactions:
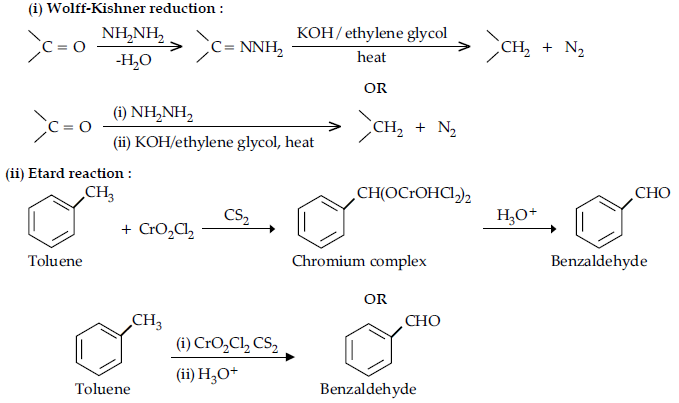
(i) Wolff-Kishner reduction
(ii) Etard reaction
Answer.
Question. Give simple chemical test to distinguish between the following pairs of compounds.
(i) Ethanal and propanal
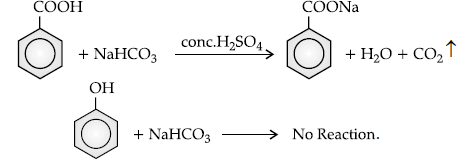
(ii) Benzoic acid and phenol.
Answer. (i) Iodoform test : Ethanal gives this test due to the presence of CH3CO group
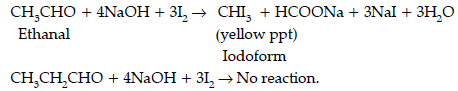
(ii) Sodium bicarbonate test : Benzoic acid gives brisk effervescence of CO2 when react with NaHCO3, while phenol does not give it, as it is a weak acid.
Question. Write the structures and IUPAC names of the cross aldol condensation products only of ethanal and propanal.
Answer. (i) 2-Methylbut-2-enal
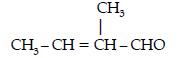
(ii) Pent-2-enal
CH3 – CH2 – CH = CH – CHO
Long Answer Type Questions-I
Question. Predict the products of the following reactions :
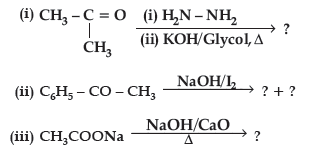
Answer. (i) CH3CH2CH3
(ii) C6H5COONa + CHI3
(iii) CH4
Question. Predict the products of the following reactions :
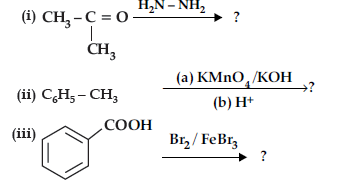
Answer. (i) (CH3)2 C = N-NH2
Question. Write the structure of the main products of following reactions :

Answer.
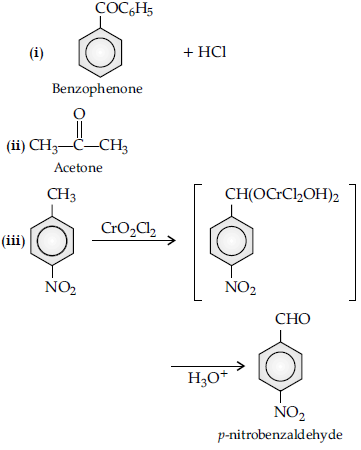
Question. How will you bring about the following conversions :
(i) Propanone to propane
(ii) Benzoyl chloride to benzaldehyde.
(iii) Ethanal to but-2-enal.
Answer.
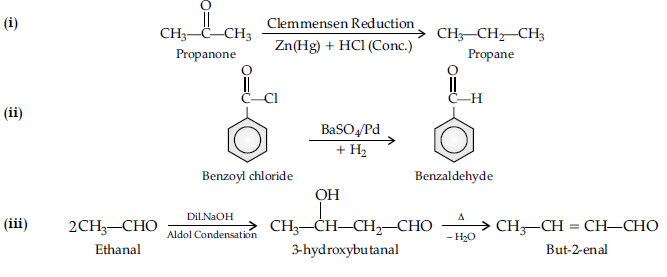
Question. (A), (B) and (C) are three non-cyclic functional isomers of a carbonyl compound with molecular formula C4H8O. Isomers (A) and (C) give positive
Tollens’ test whereas isomer (B) does not give Tollens’ test but gives positive Iodoform test.
Isomers (A) and (B) on reduction with Zn(Hg)/conc. HCl give the same product (D).
(a) Write the structures of (A), (B), (C) and (D).
(b) Out of (A), (B) and (C) isomers, which one is least reactive towards addition of HCN ?
Answer. (a) A = CH3CH2CH2CHO
B = CH3COCH2CH3
C = (CH3)2CHCHO
D = CH3CH2CH2CH3
(b) B
Long Answer Type Questions-II
Question. (i) How will you convert the following :
(a) Propanone to propan-2-ol
(b) Ethanal to 2-hydroxy propanoic acid
(c) Toluene to benzoic acid
(ii) Distinguish the following pairs of compounds :
(a) Pentan-2-one and pentan-3-one
(b) Ethanal and propanal
Answer.

Question. (i) Write the structures of A, B, C, D and E in the following reactions:
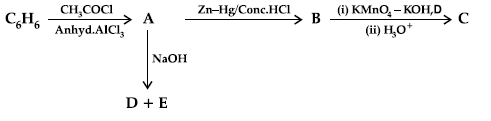
OR
(i) Write the chemical equation for the reaction involved in Cannizzaro reaction.
(ii) Draw the structure of the semicarbazone of ethanal.
(iii) Why pKa of F – CH2 – COOH is lower than that of Cl – CH2 – COOH ?
(iv) Write the product in the following reaction
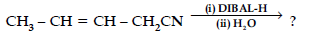
(v) How can you distinguish between propanal and propanone ?
Answer.

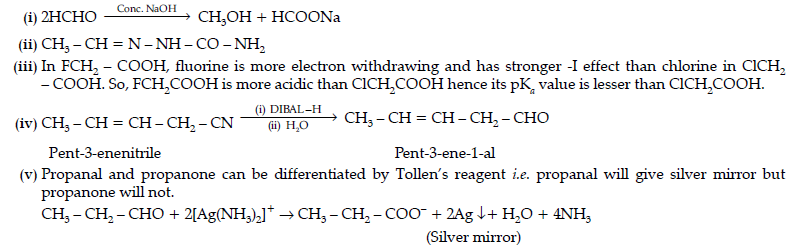
Question. (i) Write the structures of A and B in the following reactions :
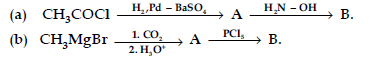
(ii) Distinguish between :
(a) C6H5 – COCH3 and C6H5 – CHO, (b) CH3COOH and HCOOH.
(iii) Arrange the following in the increasing order of their boiling points :
CH3CHO, CH3COOH, CH3CH2OH.
OR
(i) Write the chemical reaction involved in Wolff-Kishner reduction.
(ii) Arrange the following in the increasing order of their reactivity towards nucleophilic addition reaction :
C6H5COCH3, CH3 – CHO, CH3COCH3
(iii) Why carboxylic acid does not give reactions of carbonyl group ?
(iv) Write the product in the following reaction
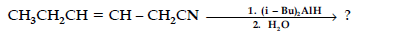
(v) A and B are two functional isomers of compound C3H6O. On heating with NaOH and I2, isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
Answer.
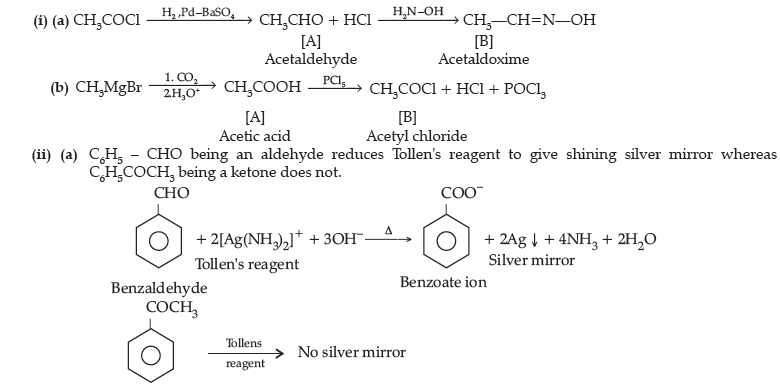
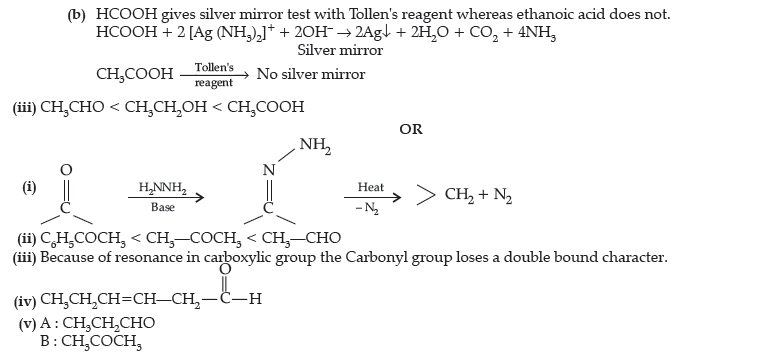
Question. (i) What is meant by the following terms ? Give an example of the reaction in each case.
(a) Aldol
(b) Semicarbazone
(ii) Complete the following :
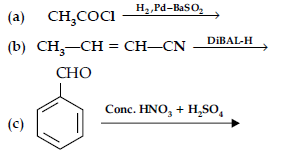
Answer. (i) (a) Aldol : Two molecules of aldehydes containing a minimum one a-hydrogen atom on treatment with dilute alkali undergo condensation to form aldol (b-hydroxy aldehydes).
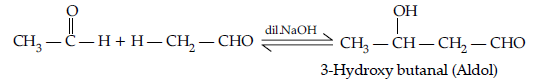
(b) Semicarbazone : Derivative of aldehydes and ketones produced by the action of semicarbazide on them in weak acid.
Topic-2
Carboxylic Acids
Very Short Answer-Objective Type Questions
A. Multiple choice Questions:
Question. Addition of water to alkynes occurs in acidic medium and in the presence of Hg2+ ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions?
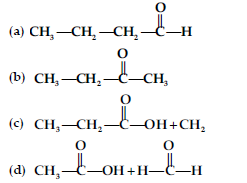
Answer
B
Question. The correct order of increasing acidic strength is :
(a) Phenol < Ethanol < Chloroacetic acid < Acetic acid
(b) Ethanol < Phenol < Chloroacetic acid < Acetic acid
(c) Ethanol < Phenol < Acetic acid < Chloroacetic acid
(d) Chloroacetic acid < Acetic acid < Phenol <
Answer
C
Question. Which is the most suitable reagent for the following conversion?
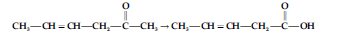
(a) Tollens’ reagent
(b) Benzoyl peroxide
(c) I2 and NaOH solution
(d) Sn and NaOH solution
Answer
A
B. Match the following:
Question. Match the species given in Column I with those mentioned in Column II.
Column I Column II
(i) Phthalic acid (a) Hexane-1,6-dioic acid
(ii) Oxalic acid (b) Benzene-1,2-dicarboxylic acid
(iii) Succinic acid (c) Pentane-1,5-dioic acid
(iv) Adipic acid (d) Butane-1,4-dioic acid
(v) Glutaric acid (e) Ethane-1,2-dioic acid
Answer. (i) → (b)
(ii) → (e)
(iii) → (d)
(iv) → (a)
(v) → (c)
C. Answer the following :
Question. Write IUPAC name of :
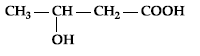
Answer. 3-Hydroxybutanoic acid/3-hydroxybutan-1-oic acid.
Question. Write the IUPAC name of :
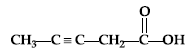
Answer. Pent-3-yne-1-oic acid.
Question. Complete the following reaction :
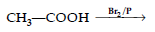
Answer.
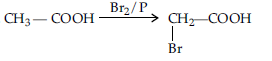
Question. Illustrate the decarboxylation reaction giving a suitable example :
Answer. Decarboxylation refers to the reaction in which carboxylic acid loose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime. e.g.,

Short Answer Type Questions
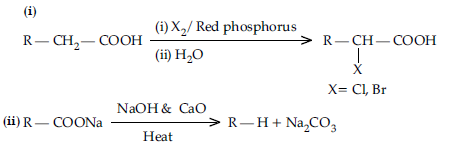
Question. Write the reactions involved in the following:
(i) Hell-Volhard Zelinsky reaction
(ii) Decarboxylation reaction
Answer.
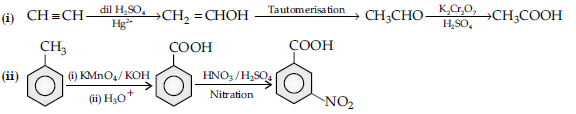
Question. How will you carry out the following conversions ?
(i) Acetylene to acetic acid
(ii) Toluene to m-nitro benzoic acid.
Answer.
Question. Although phenoxide ion has more number of resonating structures than carboxylate ion carboxylic acid is a stronger acid than phenol. Give two reasons.
Answer.

(i) Phenoxide ion has non-equivalent resonance structures in which the negative charge is at the lesser electronegative carbon atom whereas in case of carboxylate ion both the resonating structures are equivalent.
(ii) The negative charge is delocalised over two electronegative oxygen atoms in carboxylate ion whereas in phenoxide ion, the negative charge less effectively delocalises over one oxygen atom and less electronegative carbon atoms. So, the carboxylate ion is more resonance stabilised than phenoxide ion. Thus, the release of proton from carboxylic acid is much easier than from phenol. Hence, carboxylic acid is a stronger acid than phenol.
Question. Do the following conversions in not more that two steps:
(i) Propene to Acetone
(ii) Propanoic acid to 2-hydroxypropanoic acid
Answer.
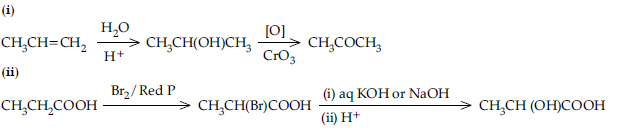
Question. How do you convert the following ?
(a) Ethanal to Propanone
(b) Toluene to Benzoic acid
OR
Account for the following :
(a) Aromatic carboxylic acids do not undergo Friedel-Crafts reaction.
(b) pKa value of 4-nitrobenzoic acid is lower than that of benzoic acid.
Answer.
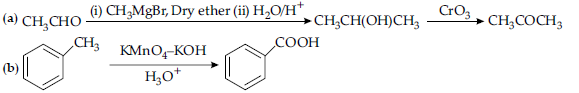
OR
(a) because the carboxyl group is deactivating and the catalyst aluminium chloride (Lewis acid) gets bonded to the carboxyl group
(b) Nitro group is an electron withdrawing group (-I effect) so it stabilises the carboxylate anion and strengthens the acid / Due to the presence of an electron withdrawing Nitro group (-I effect).
Question. Identify the reaction and write the IUPAC name of the product formed:

Answer. (a) Reaction : Hell-Volhard- Zelinsky reaction.
IUPAC : 2-Bromopropanoic acid.
(b) Reaction : Rosenmund reduction reaction.
IUPAC : Benzaldehyde.
Long Answer Type Questions
Question. Complete the following reactions :
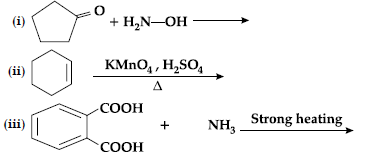
Answer.

Question. (i) Account for the following :
(a) Cl—CH2COOH is a stronger acid than CH3COOH.
(b) Carboxylic acids do not give reactions of carbonyl group.
(ii) Write the chemical equation to illustrate the following name reaction :
(a) Rosenmund reduction.
Answer. (i) (a) Cl-CH2COOH has lower pka value than acetic acid. Also, Cl group is an electron withdrawing creating less electron density on oxygen of carboxylic acid making the release of proton easier than acetate ion. Hence,Cl–CH2COOH is a stronger acid than CH3COOH.
(b) The carbonyl group in –COOH is inert and does not show nucleophilic addition reaction like carbonyl compound due to resonance stabilisation of carboxylate ion :

Question. Write structures of compounds A, B and C in each of the following reactions:
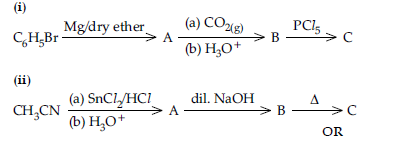
Do the following conversions in not more than two steps:
(i) Benzoic acid to benzaldehyde
(ii) Ethyl benzene to Benzoic acid
(iii) Propanone to Propene
Answer.
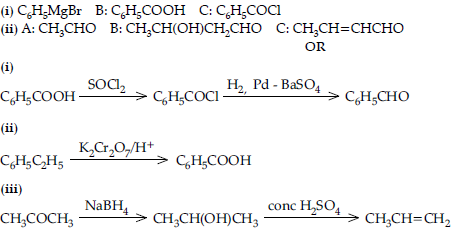
Question. Give reasons :
(i) Propanone is less reactive than ethanal towards nucleophilic addition reactions.
(ii) O2N – CH2 – COOH has lower pKa value than CH3COOH.
(iii) (CH3)2CH – CHO undergoes aldol condensation whereas (CH3)3C – CHO does not.
Answer. (i) Due to steric hindrance and +I effect caused by two alkyl groups in propanone.
(ii) Due to electron withdrawing nature of –NO2 group which increases the acidic strength and decreases the pKa value.
(iii) (CH3)2CH-CHO has one a -H atom whereas a-H atom is absent in (CH3)3C-CHO.
Question. (i) What happens when CH3 – O – CH3 is heated with HI?
(ii) Explain mechanism for hydration of acid catalyzed ethene:

Answer. (i) CH3– O– CH3+ HI → CH3–OH+CH3–I
(ii) Protonation of alkene to form carbocation by electrophilic attack of H3O+.

Question. An alcohol A (C4H10O) on oxidation with acidified potassium dichromate gives acid B(C4H8O2). Compound A when dehydrated with conc. H2SO4 at 443 K gives compound C. Treatment of C with aqueous H2SO4 gives compound D (C4H10O) which is an isomer of A. Compound D is resistant to oxidation but compound A can be easily oxidised. Identify A, B, C and D. Name the type of isomerism exhibited by A and D.
Answer.
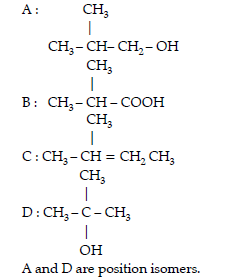
Long Answer Type Questions-II
Question. (i) Complete the following equations :
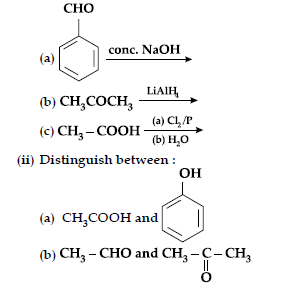
Answer.
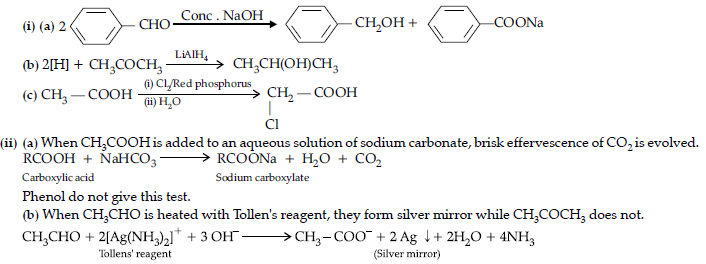
Question. (i) Describe the following giving chemical equations :
(a) De-carboxylation reaction
(b) Friedel-Crafts reaction
(ii) How will you bring about the following conversions ?
(a) Benzoic acid to Benzaldehyde
(b) Benzene to m-Nitroacetophenone
(c) Ethanol to 3-Hydroxybutanal
OR
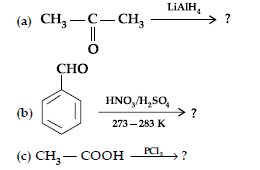
(i) Describe the following reactions :
(a) Acetylation (b) Aldol condensation
(ii) Write the main product in the following equations :
Answer. (i) (a) Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime (NaOH and CaO).

(b) When the alkyl / acyl group is introduced at ortho and para positions by reaction with alkyl halide / acyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as catalyst.
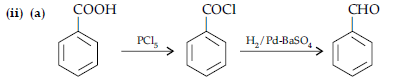
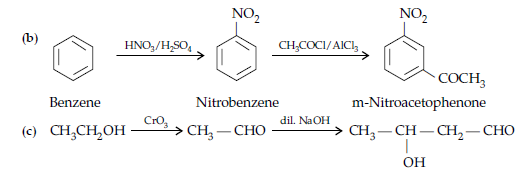
OR
(i) (a) When the acyl groups are introduced at ortho and para positions by reaction with acyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as catalyst.
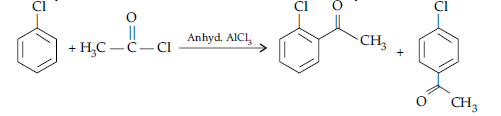
(b) Aldehydes and ketones having at least one a-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to form b-hydroxy aldehydes (aldol) or b-hydroxy ketones (ketol), respectively.
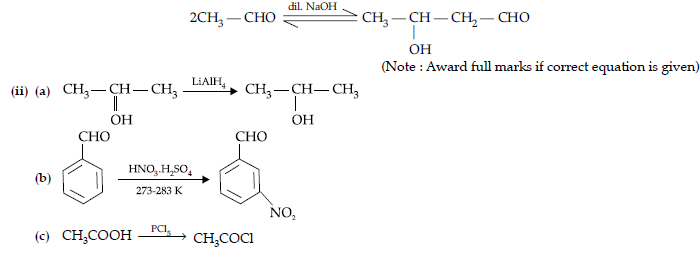
Question. (i) Draw the structures of the following :
(a) p-Methylbenzaldehyde,
(b) 4-Methylpent-3-en-2-one.
(ii) Give chemical tests to distinguish between the following pairs of compounds :
(a) Benzoic acid and Ethyl benzoate,
(b) Benzaldehyde and Acetophenone
(c) Phenol and Benzoic acid.
OR
(i) Draw the structures of the following derivatives :
(a) Propanone oxime,
(b) Semicarbazone of CH3CHO.
(ii) How will you convert ethanal into the following compounds ? Give the chemical equations involved.
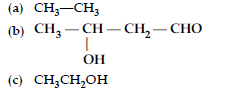
Answer.

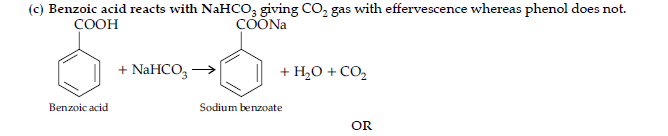
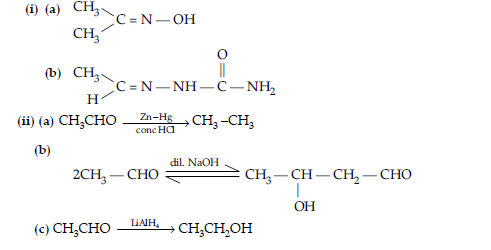
Question. (i) Write the products of the following reactions :
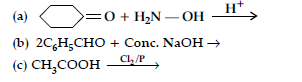
(ii) Give, simple chemical tests to distinguish between the following pairs of compounds :
(a) Benzaldehyde and benzoic acid
(b) Propanal and propanone.
Answer.

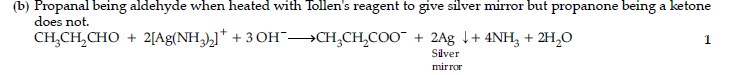
Question. (i) Account for the following :
(a) CH3CHO is more reactive than CH3COCH3 towards reaction with HCN.
(b) Carboxylic acid is stronger acid than phenol.
(ii) Write chemical reactions to illustrate the following name reactions :
(a) Wolff Kishner reduction
(b) Aldol condensation
(c) Cannizzaro reaction.
Answer. (i) (a) Because the positive charge on carbonyl carbon of CH3CHO decreases to a lesser extent due to one electron releasing (+I effect) CH3 group as compared to CH3COCH3 (two electron releasing CH3 group) and hence more reactive.
(b) Because carboxylate ion (conjugate base) is more resonance stabilized than phenoxide ion.
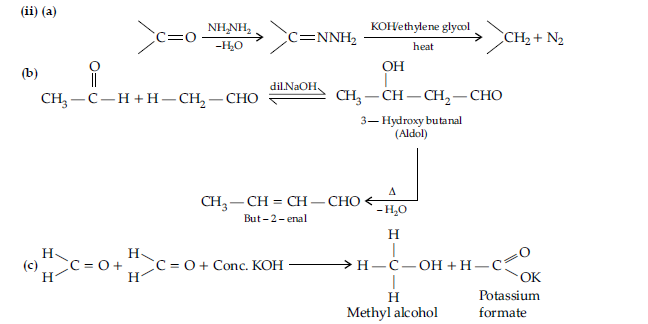
Question. (i) Illustrate the following name reactions giving suitable example in each case :
(a) Clemmensen reduction
(b) Hell Volhard-Zelinsky reaction.
(ii) How are the following conversions carried out ?
(a) Ethylcyanide to ethanoic acid,
(b) Butan-1- ol to butanoic acid,
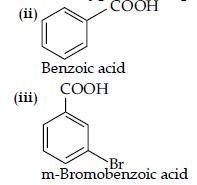
(c) Benzoic acid to m-bromobenzoic acid.
Answer. (i) (a) Clemmensen reduction : The carbonyl group of aldehydes and ketones is reduced to—CH2 group on treatment with zinc amalgam and concentrated HCl
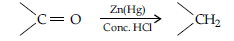
(b) Hell Volhard-Zelinsky reaction : Carboxylic acids having an a-hydrogen are halogenated at the a-position on treatment with chlorine or bromine in the presence of red phosphorus to give a-halocarboxylic acids.
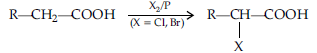
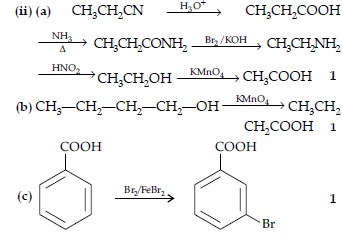
Question. (i) Write a suitable chemical equation to complete each of the following transformations :
(a) Butan-1-ol to butanoic acid
(b) 4-Methyl acetophenone to benzene-1, 4-dicarboxylic acid
(ii) An organic compound with molecular formula C9H10O forms 2,4-DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro’s reaction. On vigorous oxidation it gives 1,2-benzene dicarboxylic acid. Identify the compound.
Answer.
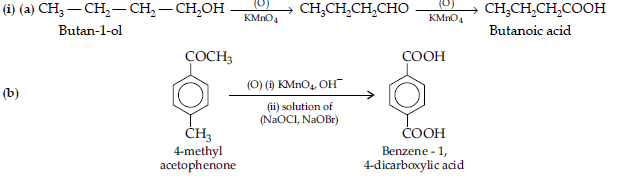
(ii) (a) It is an aldehyde or ketone as it forms 2, 4-DNP derivative.
(b) As the compound reduces Tollen’s reagent and undergoes Cannizzaro reaction, it is an aldehyde and not a ketone.
(c) On vigrous oxidation, it gives 1, 2-benzenedicarboxylic acid. So, it must have an alkyl group at ortho position with respect to CHO group on the benzene ring.
(d) Molecular formula suggests that it should be 2-ethyl benzaldehyde.
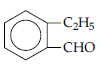

Question. (i) Give a plausible explanation for each one of the following :
(a) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol.
(b) There are two -NH2 groups in semicarbazide.
However, only one is involved in the formation of semicarbazones.
(ii) Carry out the following conversions in not more than two steps :
(a) Phenyl magnesium bromide to benzoic acid.
(b) Acetaldehyde to But-2-enal.
(c) Benzene to m-Nitroacetophenone.
OR
(i) Give a simple chemical test to distinguish between the pair of organic compounds :
Ethanal and Propanal
(ii) Name and complete the following chemical reaction :
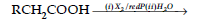
(iii) Draw the structures of the following derivatives :
(a) The 2, 4-Dinitrophenylhydrazone of benzaldehyde,
(b) Acetaldehyde dimethyl acetal
(c) Cyclopropanone oxime.
Answer. (i) (a) The delocalisation of benzene electrons contributes little towards the stability of phenoxide ion. The carboxylate ion is much more resonance stabilized than phenoxide ion. So,it is easier to lose a proton than phenol. Hence, carboxylic acid is a stronger acid than phenol.
(b) Semicarbazide has two —NH2 groups. One of them, which is directly attached to C=O is involved in resonance. Thus, electron density on this group decreases and it does not act as a nucleophile. In contrast, the lone pair of electrons on the other —NH2 group is available for nucleophilic attack.
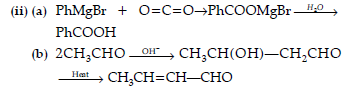
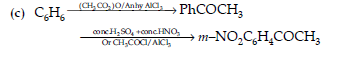
OR
(i) Ethanal and propanal can be distinguished by Iodoform test.
Ethanal gives a yellow precipitate of iodoform with an alkaline solution of NaOH. Propanal does not gives this test.
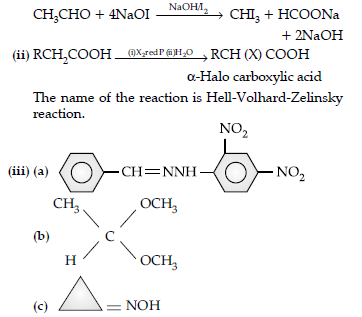
Question. (i) Write the product(s) in the following reactions:


(ii) Give simple chemical tests to distinguish between the following pairs of compounds:
(a) Ethanol and Phenol
(b) Propanol and 2-methylpropan -2-ol
Answer.
(b) (CH3)2 CHOH and CH3 CH2I
(c) CH3CH=CHCHO
(ii) (a) Add neutral FeCl3 to both the compounds, phenol gives violet complex.
(b) Add anhy ZnCl2 and conc. HCl to both the compounds, 2-methyl propan-2-ol gives turbidity immediately.
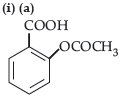
Question. (i) Write the product(s) in the following reactions :
(ii) Give simple chemical tests to distinguish between the following pairs of compounds:
(a) Butanal and Butan-2-one
(b) Benzoic acid and Phenol
OR
(i) Write the reactions involved in the following:
(a) Etard reaction
(b) Stephen reduction
(ii) How will you convert the following in not more than two steps :
(a) Benzoic acid to Benzaldehyde
(b) Acetophenone to Benzoic acid
(c) Ethanoic acid to 2-Hydroxyethanoic acid
Answer.
(c) CH3–CH=CH–CHO
(ii) (a) Tollen’s reagent test : Add ammoniacal solution of silver nitrate (Tollen’s Reagent) in both the solutions.
Butanal gives silver mirror whereas Butan-2-one does not.
(b) Add neutral FeCl3 in both the solution, phenol forms violet colour but benzoic acid does not.
(or any other correct test)
OR
(i) (a) Etard reaction

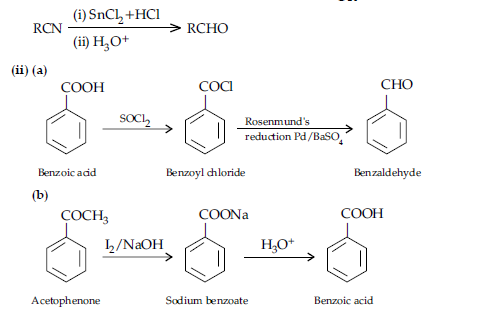

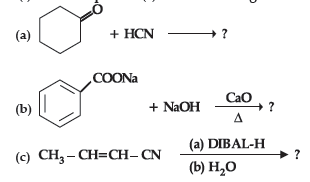
Question. (i) Account for the following :
(a) Propanal is more reactive than propanone towards nucleophilic reagents.
(b) Electrophilic substitution in benzoic acid takes place at meta position.
(c) Carboxylic acids do not give characteristic reactions of carbonyl group.
(ii) Give simple chemical test to distinguish between the following pairs of compounds:
(a) Acetophenone and benzaldehyde
(b) Benzoic acid and ethylbenzoate
OR
(i) Write structures of A, B, C and D in the following reaction sequence:
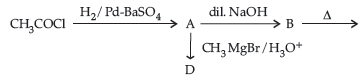
(ii) Arrange the following compounds in the increasing order of their boiling points :
CH3CHO, CH3CH2OH, CH3OCH3, CH3COOH
Answer. (i) (a) Due to steric and +I effect of two methyl groups in propanone.
(b) Because it is a deactivating group/Due to electron withdrawing carboxylic group resulting in decreased electron density at o- and p- position.
(c) Due to resonance, electrophilicity of carbonyl carbon is reduced.
(ii) (a) Add NaOH and I2 to both the compounds and heat, acetophenone forms yellow ppt of iodoform.
(b) Add NaHCO3 solution to both the compounds,benzoic acid will give effervescence and liberates CO2.
OR
(i) A: CH3CHO; B: CH3-CH(OH)-CH2-CHO; C: CH3-CH=CH-CHO; D: CH3-CH(CH3)-OH
(ii) CH3-O-CH3<CH3CHO<CH3-CH2-OH < CH3-COOH
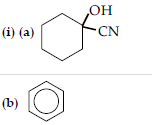
Question. (i) How will you convert:
(a) Benzene to acetophenone
(b) Propanone to 2-Methylpropan-2-ol
(ii) Give reasons:
(a) Electrophilic substitution in benzoic acid take place at meta position.
(b) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.
(c) Propanal is more reactive than propanone in nucleophilic addition reactions.
OR
(i) Write the products of the following reaction:
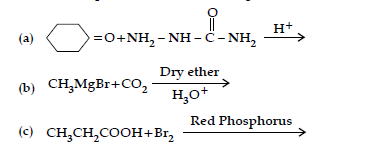
(ii) Write simple chemical tests to distinguish between the following pairs of compounds.
(a) Propanal and propanone
(b) Benzaldehyde and Benzoic acid
Answer.
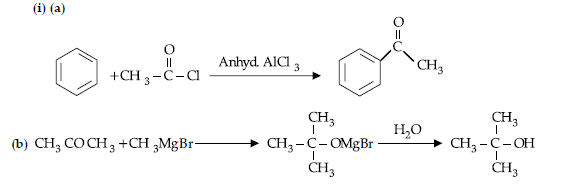
(ii) (a) Because it is a deactivating group/Due to electron withdrawing carboxylic group resulting in decreased electron density at o- and p- position.
(b) Due to extensive association of carboxylic acid molecules through intermolecular hydrogen bonding.
(c) Due to steric and +I effect of two methyl groups in propanone.
OR
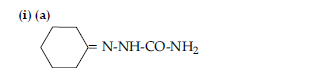
(b) CH3 COOH
(c)CH3 — CH(Br)— COOH
(ii) (a) Add ammonical solution of silver nitrate / Tollen’s reagent to both the compounds, propanal will give silver mirror while propanone does not.
(b) Add NaHCO3 solution to both the compounds, benzoic acid will give effervescence and liberate CO2 while benzaldehyde will not.
Question. (i) Write the products of the following reactions :
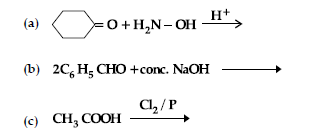
(ii) Give simple chemical tests to distinguish between the following pairs of compounds:
(a) Benzaldehyde and Benzoic acid
(b) Propanal and Propanone
Answer.

(c) Cl–CH2–COOH
(ii) (a) NaHCO3 test.
(b) lodoform test./Fehling’s Test/Tollen’s Test
Question. (i) Give reasons :
(a) HCHO is more reactive than CH3 – CHO towards addition of HCN.
(b) pKa of O2N – CH2 – COOH is lower than that of CH3 – COOH.
(c) Alpha hydrogen of aldehydes & ketones is acidic in nature.
(ii) Give simple chemical tests to distinguish between the following pairs of compounds :
(a) Ethanal and Propanal
(b) Pentan-2-one and Pentan-3-one
OR
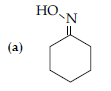
(i) Write structure of the product(s) formed :
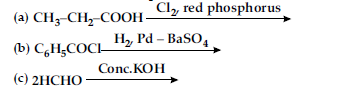
(ii) How will you bring the following conversions in not more than two steps :
(a) Propanone to propene
(b) Benzyl chloride to phenyl ethanoic acid
Answer. (i) (a) Due to +I effect of methyl group in CH3CHO.
(b) due to –I effect of nitro group in nitroacetic acid.
(c) Due to the strong electron withdrawing effect of the carbonyl group and resonance stabilisation of the conjugate base.
(ii) (a) Add NaOH and I2 to both the compounds and heat, ethanal gives yellow ppt of iodoform.
(b) Add NaOH and I2 to both the compounds and heat, pentan-2-one gives yellow ppt of iodoform.
OR
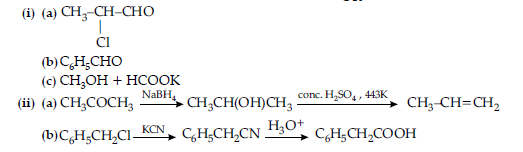
Question. An aromatic compound ‘A‘ of molecular formula C7H6O2 undergoes a series of reactions as shown below.
Write the structures of A, B, C, D and E in the following reactions :
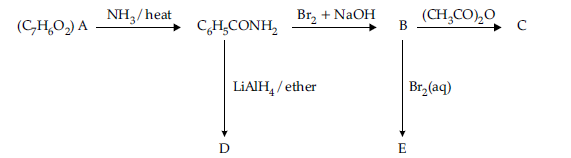
Answer.