Please refer to Carbon and Its Compound Class 10 Science Important Questions given below. These solved questions for Carbon and Its Compound have been prepared based on the latest CBSE, NCERT and KVS syllabus and books issued for the current academic year. We have provided important examination questions for Class 10 Science all chapters.
Class 10 Science Carbon and Its Compound Important Questions
VERY SHORT ANSWER TYPE QUESTIONS :
Question. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer : (b) The fuel is not burning completely.
Question. Write the name and formula of the first member of the carbon compounds having functional group —COOH.
Answer.

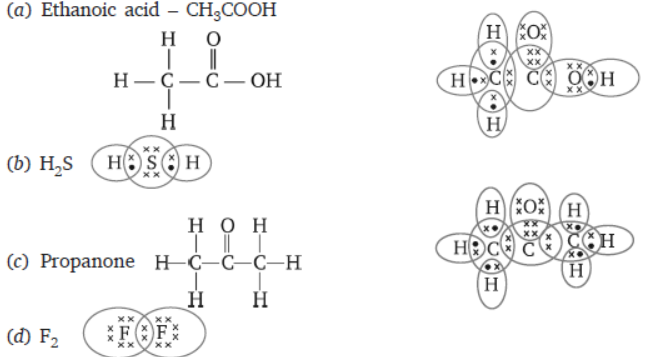
Question. Draw the electron dot structure for
(a) ethanoic acid.
(b) H2S.
(c) propanone.
(d) F2.
Answer : The electron dot structure are as follows:
Question. Write the name and formula of the second member of the carbon compounds having functional group —OH.
Answer.

Question. What change will you observe if you test soap with litmus paper (red and blue)?
Answer : Soap is alkaline in nature, hence it will turn red litmus into blue, blue litmus will remain blue.
Question. Write the name and formula of the 2nd member of the series of carbon compounds whose general formula is CnH2n+1OH
Answer. Ethanol, C2H5OH or CH3CH2OH
Question. Write the name and formula of the 2nd member of the series of carbon compounds whose general formula is CnH2n.
Answer.
C3H6, H2C=CH—CH3
Propene is second member of series whose general formula is CnH2n.
Question. Write the name and formula of the first member of the carbon compounds having functional group —CHO.
Answer.

Question. What is meant by a sturated hydrocarbon?
Answer: Those hydrocarbons in w hich valency of carbon is satisfied by single bonds only are called sturated hydrocarbons.
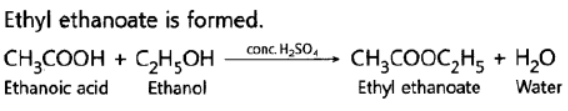
Question. Name the compound formed when ethanol is warmed with ethanoic acid in the presence of a few drops of cone.H2S04
Answer:
Question. What happens when a small piece of sodium is dropped into ethanol?
Answer: Hydrogen gas will be evolved.
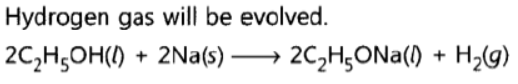
Question. State two characteristic features of carbon which when put together give rise to large number of carbon compounds.
Answer: (i) Catenation (ii) Tetravalency of carbon
Question. Write the structural formula of chloroethane.
Answer:

Question. How many covalent bonds are there in a molecule of ethane (C2H6)?
Answer: There are 7 covalent bonds in a molecule of ethane.
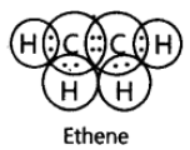
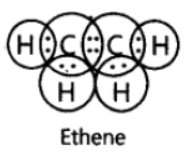
Question. Write the electron dot structure of ethene molecule (C2H4).
Answer:
Question. What is the difference in the molecular formula of any two consecutive members of a homologous series of organic compounds?
Answer: —CH2— is the difference in the molecular formula of any two consecutive members of a homologous series of organic compounds.
Question. Name the carbon compound which on heating with excess of concentrated sulphuric acid at 443 K gives ethene.
Answer:
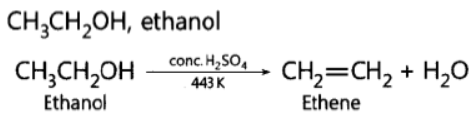
Question. Write the name and formula of the first member of the carbon compounds having functional group —COOH.
Answer:
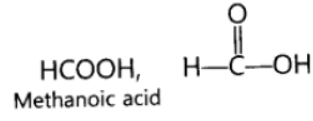
Question. Write the electron dot structure of ethane molecule (C2H6).
Answer:
Question. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer : Physical Properties
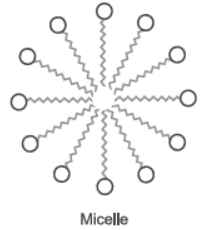
Question. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer : Soap molecules have two ends with different properties. One end is hydrophilic, which dissolves in water and other end is hydrophobic, it dissolves in hydrocarbons. When soap is added to water, the ionic end of soap will form a unique orientation and keep the hydrocarbon tail away from it.
The cluster of molecules is formed in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. Hence, micelle formation takes place.
Soap is soluble in ethanol hence the micelle formation will not take place.
Question. Why are carbon and its compounds used as fuels for most applications?
Answer : Carbon and its compounds undergo combustion to produce heat, the amount of heat released can be handled and used so they are used as fuels for most applications.
Question. Explain the formation of scum when hard water is treated with soap.
Answer : Hard water contains salts of calcium and magnesium. When soap molecule comes in contact with these salts it forms a curdy white precipitate (compound insoluble in water) called scum.
Soap + Hard water ⎯→ scum
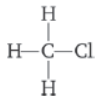
Question. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer : Bond formation in CH3Cl
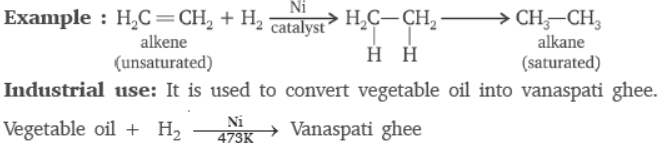
Question. What is hydrogenation? What is its industrial application?
Answer : When unsaturated hydrocarbons (double/triple bond) are reacted with hydrogen in presence of a catalyst like nickel, the hydrogen gets added across the double/triple bond and converts the unsaturated hydrocarbon into saturated hydrocarbon. Such reaction is called addition reaction or hydrogenation.
Question. What is catenation?
Answer : Carbon has the unique ability to form bonds with the other atoms of carbon which gives rise to large molecules. This property of self linking is called catenation.
Question. Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer : Butter is saturated compound and oil is unsaturated compound.
Test alk. potassium permanganate + Unsaturated → Pink colour disappear.
(Pink colour) hydrocarbon
Therefore, when we add oil to a test tube containing alkaline potassium permanganate solution, the pink colour of the solution disappear. Colour of alkaline potassium permanganate will not disappear in the test tube containing butter.
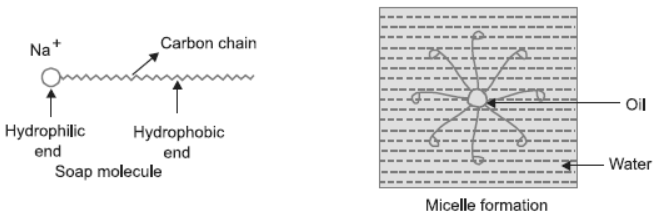
Question. Explain the mechanism of cleaning action of soaps.
Answer : Soap molecule has two ends, the charged end that gets attracted towards water is called hydrophilic and the long carbon chain that repels water is called hydrophobic end. When soap is dissolved in water, the carbon chain i.e., hydrophobic end gets attracted towards the oil, dirt and grease. The hydrophilic end stays away from this.
The micelle formation takes place.
The tail entangles dirt, oil or grease, if required the agitation is done. Lot of rinsing is a done with water so that water molecules attract charged (Na+) end and carries the soap molecules with dirt attached to it and clean the clothes, utensils, etc.
Question. Which of the following hydrocarbons undergo addition reactions?
C2H6, C3H8, C3H6, C2H2 and CH4.
Answer : Addition reaction takes place in unsaturated hydrocarbons.
Hence C3H6 and C2H2 are unsaturated hydrocarbons and will show addition reaction.
Question. Give two properties of ionic compounds.
Answer : (i) High melting point and high boiling point.
(ii) Can conduct electricity.
Question. What is the melting point of acetic acid?
Answer : M.P. = 290 K.
Question. Name the given compound
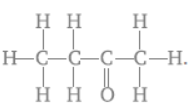
Answer : 2-Butanone.
Question. Name the compound
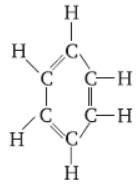
Answer : Benzene, C6H6.
Question. How can you convert ethene into ethane?
Answer : By adding hydrogen to ethene in the presence of a catalyst.
Question. Why can’t we test hard water with detergents?
Answer : Detergents form lather with both hard and soft water hence we cannot distinguish between them.
Question. What is esterification reaction?
Answer : The reaction in which alcohol reacts with carboxylic acid to produce a new compound called ester is called esterification.
Question. Give two uses of methane gas.
Answer : (i) It is used as a fuel (ii) It is the major component of biogas and CNG.
Question. What is isomerism?
Answer : A property in which a compound can exist in different structural formula but its molecular formula remains the same.
Question. What is hydrophilic?
Answer : The substance showing attraction towards water is called hydrophilic.
Question. A compound has a molecular formula C2H6O. It is used as a fuel. Name the compound and name its functional group.
Answer : C2H6O is an alcohol, i.e. ethanol C2H5OH
Functional group is —OH.
Question. Name the second member of alkyne series.
Answer : Propyne
Question. Give the names of the functional group
(i) —CHO (ii) —C==O
Answer : (i) —CHO → Aldehyde (ii) (Img 28)
Question. The structural formula of an ester is (Img 28)
Name the alcohol and the acid from which it would have been formed.
Answer : Alcohol is C2H5OH ethanol
Acid is H3C—H2C—COOH propanoic acid.
Question. Give the IUPAC name of acetic acid and propyl alcohol.
Answer : Acetic acid – Ethanoic acid
Propyl alcohol – Propanol
Question. What will happen to the litmus solution in carboxylic acid?
Answer : Red litmus remains the same but blue litmus changes to red.
Question. Give the electron dot structure of CH3Cl and C2H2.

Answer :

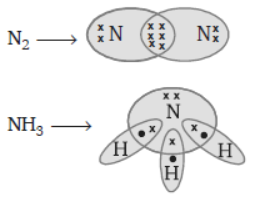
Question. Draw the electron dot structure of N2 and NH3.
Answer :
Question. Why do soaps form scum when added to hard water?
Answer : Hard water contains carbonate and sulphate salts of magnesium or sodium ions which react with the soap molecule to form a compound which is insoluble in water. Hence soaps form scum with hard water.
Question. What happens when ethanol burns in air?
Answer : Ethanol burns to form carbon dioxide and water.
SHORT ANSWER TYPE QUESTIONS
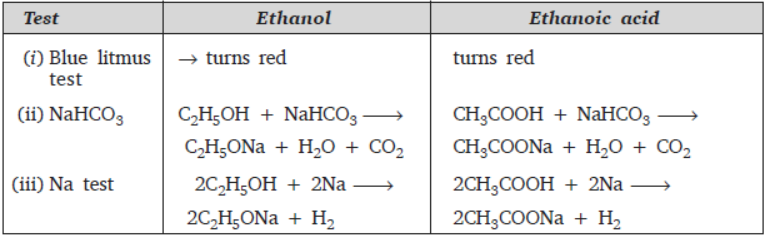
Question. Differentiate between ethanol and ethanoic acid on basis of the following test:
(i) Blue litmus test (ii) Reaction with sodium bicarbonate (iii) Sodium metal test
Answer :
Question. (a) Name the compound CH3CH2OH and identify its functional group.
(b) Give a chemical test to distinguish between ethanol and ethanoic acid.
(c) Name the product formed when an organic acid reacts with an alcohol in presence of an acid catalyst. What is the name assigned to this type of reaction? (AI CBSE 2008 C)
Answer : (a) CH3CH2OH – Ethanol
Functional group: alcohol (–OH)
Question. Giving chemical equations of the reactions write what happens when
(i) Ethanol is heated with excess of concentrated sulphuric acid at 443 K.
(ii) Ethanoic acid reacts with ethanol in presence of an acid.
(iii) Ester with molecular formula CH3COOC2H5 reacts with sodium hydroxide.
Answer :
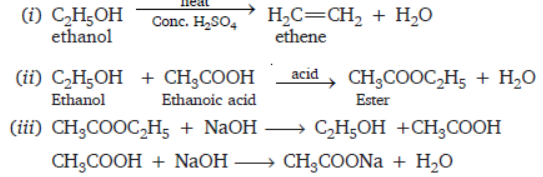
Question. Why does carbon forms large number of compounds?
Answer : Carbon forms large number of compounds because of tetravalency and catenation property.
Tetravalency – Carbon has valency 4, to attain noble gas configuration carbon share its valence electrons with other elements like hydrogen, chlorine, etc.
Catenation – Carbon also shows the property of self-linking in which it forms long, branched or cyclic chains to form large number of compounds.
Question. How can you obtain the following from pure ethanol:
(i) Ethene (ii) Ethanoic acid (iii) Ester?
Answer : (i) Ethene: Ethanol when heated with excess of concentrated sulphuric acid will form ethene.
Question. (a) What is a ‘homologous series’ of substances?
(b) In an organic compound, which parts largely determine its physical and chemical properties?
(c) Write a chemical equation to represent the reaction of ethanol with acidified solution of potassium dichromate.
Answer : (a) Homologous series is a series of organic compounds having same general formula, all members of the compounds show same chemical properties and slight gradation in physical properties.
(b) Functional group.
Question. An organic compound ‘X’ which is also called antifree mixture has the molecular formula C2H6O ‘X’ on oxidation gives a compound ‘Y’ which gives effervescence with a baking soda solution. What can X and Y be? Write their structural formula.
Answer : X is ethanol, (C2H5OH)
Y is ethanoic acid (CH3COOH)

Question. Write the structures of isomers of hexane.
Answer :

Question. Complete and balance the following equations:
(a) CH3CH2OH + O2 ⎯→
(b) Na + CH3CH2OH ⎯→
Answer : (a) 2CH3CH2OH + 6O2 ⎯⎯→ 4CO2 + 6H2O + heat + light
(b) 2Na + 2CH3CH2OH ⎯⎯→ 2CH3CH2ONa + H2
Question. Give two uses of ethanol and one harmful effect of it.
Answer : Ethanol is a good solvent so it is used in making medicines such as tincture iodine, cough syrups and many tonics. Ethanol is also used in making alcoholic drinks.
Harmful effects: Intake of small amount of ethanol leads to drunkenness. Intake of even small amount of ethanol can be lethal. Long-term use or consumption can lead to severe health problems.
Question. (a) Name the compound CH3COOH and identify its functional group.
(b) Give a chemical test to identify this compound.
(c) Name the gas evolved when this compound acts on solid sodium carbonate. How would you identify this gas? (AI CBSE 2008 C)
Answer : (a) Ethanoic acid, functional group is—COOH (Caboxylic/group)
(b) Take few drops of ethanoic acid in a test tube and add sodium hydrogen carbonate solution to it. Brisk effervescence of CO2 gas is formed.
(c) CO2 gas is evolved. To identify the gas, pass it through freshly prepared limewater, it turns milky due to the formation of milky white precipitate of CaCO3.
Ca(OH)2 + CO2 ⎯⎯→ CaCO3 + H2O
Question. (a) Give a chemical test to distinguish between saturated and unsaturated hydrocarbons.
(b) (i) Name the products formed when ethanol burns in air.
(ii) What two forms of energy are liberated on burning alcohol?
(c) Why is the reaction between methane and chlorine considered a substitution reaction?
Answer : (a) On adding bromine water, the unsaturated hydrocarbon decolourises the bromine water but the saturated hydrocarbon will not decolourise bromine water.
(b) (i) Ethanol burns in air to produce carbon dioxide and water.
C2H5OH + 3O2 ⎯⎯→ 3CO2 + 3H2O + heat
(ii) Two forms of energy obtained are heat energy and light energy.
(c) When methane reacts with chlorine, the hydrogen atom of methane is replaced by chlorine atom step by step and hence it is termed as substitution reaction.
CH4 + Cl2 ⎯⎯→ CH3Cl + HCl
Question. Name the peculiar/specific chemical property exclusive in case of saturated hydrocarbons and unsaturated hydrocarbons.
Answer : Saturated hydrocarbons show substitution reaction in which hydrogen atom gets substituted by other elements or atoms. Unsaturated hydrocarbons show addition reaction, in which hydrogen atom gets added across the double bond or triple bond of the compound.
Question. Give reason for the following observations:
(a) The element carbon forms a very large number of compounds.
(b) Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame.
(c) Use of synthetic detergents causes pollution of water.
Answer : (a) Carbon forms large number of compounds due to its property of catenation, i.e. self linking. They form isomeric compounds i.e. compounds with same molecular formula but different structural formula.
(b) The vessels blacken due to deposits of black carbon particles on it which is caused due to incomplete combustion of fuel. Air holes are adjusted so that air enters through the holes and helps in complete combustion of the fuel.
(c) Synthetic detergent is non-biodegradable, it remains in the water thereby causing water pollution.
Question. What is meant by a functional group in an organic compound? Name the functional group present in
(i) CH3CH2OH
(ii) CH3COOH
(b) State one point of difference between soap and synthetic detergent.
Answer:
(a) Functional group is an atom or group of atoms or reactive part of compound, which determines chemical properties of compounds.
(i) —OH (Alcohol)
(ii) —COOH (Carboxylic acid)
(b) Soaps do not work well with hard water, detergents work well with hard water.
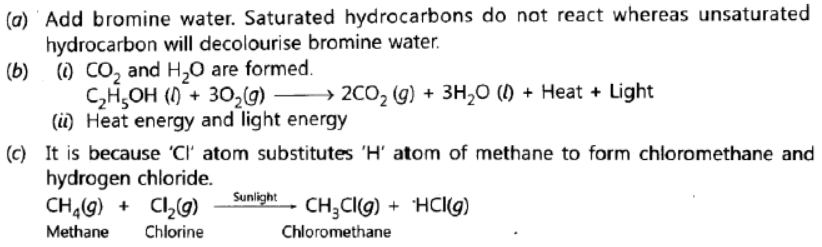
Question.
(a) Give a chemical test to distinguish between saturated and unsaturated hydrocarbons.
(b) (i) Name the products formed when ethanol burns in air. ‘
(ii) What two forms of energy are liberated on burning alcohol?
(c) Why is the reaction between methane and chlorine considered a substitution reaction?
Answer:
Question.
(a) Why are covalent compounds generally poor conductors of electricity?
(b) Name the following compound:
(c) Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Answer:

Question. Give reasons for the following observations:
(a) The element carbon forms a very large number of compounds.
(b) Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame.
(c) Use of synthetic detergents causes pollution of water.
Answer:
(a) Carbon forms large number of compounds since carbon is small in size and can form stable covalent bonds (catenation) and it shows tetravalency.
(b) Air holes of gas burner are made open (adjusted) so that air can pass through, which is needed for complete combustion, so that heated vessels do not get blackened.
(c) Some synthetic detergents are non-biodegradable, therefore, cause pollution of water.
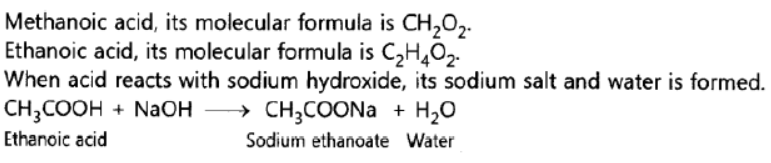
Question. What is ethanoic acid? Write the formula of the functional group present in this acid.
What special name is given to its 5 – 8% solution in water? How does ethanoic acid react with sodium carbonate? Write a chemical equation of the reaction and common name of the salt produced.
Answer:

Question. Write the names and molecular formula of two organic compounds having functional r group suffixed as ‘-oic acid’. With the help of a balanced chemical equation and explain what happens when any one of them reacts with sodium hydroxide.
Answer:
Question. What is a homologous series? Which two of the following organic compounds belong to the same homologous?
CH3 ,C2H6, C2H6O, C2H6O2,CH4O
Answer:

Question. Name the functional group of organic compounds that can be hydrogenated. With the help of suitable example explain the process of hydrogenation mentioning the conditions of the reaction and any one change in physical property with the formation of the product. Name any one natural source of organic compounds that are hydrogenated.
Answer:
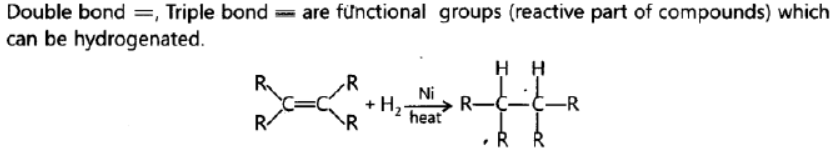
When unsaturated hydrocarbons are heated with hydrogen in the presence of nickel as catalyst, saturated hydrocarbons are formed. If the starting unsaturated hydrocarbons are liquids, they will change into solids. Vegetable oils are hydrogenated to form vegetable ghee. Plants are natural sources of vegetable oils which can be hydrogenated.
Question. Write the name and molecular formula of an organic compound having its name suffixed with ‘-ol and having two carbon atoms in the molecule. With the help of a balanced chemical equation indicate what happens when it is heated with excess of r cone.H2S04.
Answer:

Question. With the help of balanced chemical equations explain what happens when ethanol is heated with (i) alkaline solution of potassium permanganate, (ii) excess concentrated sulphuric acid at 443 K. Mention any two uses of ethanol.
Answer:

Question. (a) What is vinegar?
(b) Describe with a chemical equation, what happens when sodium hydrogen carbonate reacts with ethanoic acid. (AI CBSE 2009)
Answer : (a) The 5% – 10% aqueous solution of acetic acid is called vinegar.
(b) CH3COOH + NaHCO3 ⎯⎯→ CH3COONa + H2O + CO2
Acetic acid Sodium acetate
Ethanoic acid reacts with sodium bicarbonate to produce brisk effervescence of CO2 gas and sodium acetate.
Question. (a) Write the names of the functional groups in: (Img 37)
(b) Describe a chemical test to distinguish between ethanol and ethanoic acid.
Answer : (a) (i) Ketone (ii) Aldehyde
(b) On adding Na2CO3/NaHCO3, the test tube containing ethanoic acid produces brisk effervescence of CO2 gas. Alcohol will not show any reaction.
CH3COOH + NaHCO3 ⎯→ CH3COONa + CO2 + H2O
C2H5OH + NaHCO3 ⎯→ No reaction
LONG ANSWER TYPE QUESTIONS
Question. (a) Why does carbon form compounds mainly by covalent bonding?
(b) List any two reasons for carbon forming a very large number of compounds.
(c) An organic acid ‘X’ is a liquid which often freezes during winter time in cold countries, has the molecular formula, C2H4O2. On warming it with ethanol in the presence of a few drops of concentrated sulphuric acid, a compound ‘Y’ with a sweet smell is formed.
(i) Identify ‘X’ and ‘Y’.
(ii) Write a chemical equation for the reaction involved.
Answer : (a) Carbon forms compounds mainly by covalent bonding because carbon has small size, so neither it can loose four electrons easily, because very high amount of energy will be required, nor it can gain four electrons. Hence, it shares four electrons forming covalent bonds.
(b) (i) Due to catenation – Self linking property
(ii) Tetravalency of carbon – Forms compounds with other elements.
(c) (i) ‘X’ is CH3COOH, it freezes during winter in cold countries.
‘Y’ is ester CH3COOC2H5
(ii) CH3COOH + C2H5OH ⎯C⎯onc⎯. H2SO4→ CH3COOC2H5 + H2O
Ethanoic acid Ethanol Ester
Question. (a) What is homologous series of compounds? List any two characteristics of a homologous series.
(b) (i) What would be observed on adding 5% solution of alkaline potassium permanganate solution drop by drop to some warm ethanol taken in a test tube?
(ii) Write the name of the compound formed during the chemical reaction.
(c) How would you distinguish experimentally between an alcohol and a carboxylic acid on the basis of a chemical property?
Answer : (a) Organic compounds when arranged in series having same general formula and similar chemical properties is called homologous series.
Two characteristics of homologous series.
(i) Each successive member differ by —CH2 group, 14 u.
(ii) The method of preparation and chemical properties of members of homologous series is same.
(b) (i) The colour of KMnO4 will get discharged because ethanol gets oxidised to form ethanoic acid.
CH3CH2OH + 2(O) ⎯a⎯lk. ⎯KMnO4→ CH3COOC2H5 + H2O
(ii) Ethanoic acid
(c) On adding sodium bicarbonate solution to both the test tubes containing ethanol and ethanoic acid then the test tube containing carboxylic acid (ethanoic acid) will show brisk effervescence due to formation of CO2 gas. Alcohol will not react with sodium bicarbonate.
Question. (a) What is a functional group in a carbon compound? Identify the functional group present in CH3COOH and C2H5OH.
(b) State the principle on which the cleansing action of soap is based.
Answer : (a) The atom or group of atoms which determines the properties of a compound is called functional group.
— OH is alcohol group
— COOH is carboxylic group
(b) Cleansing action of soap depends on its structure, it has two ends hydrophobic which attracts dirt, oil or grease and hydrophilic end which attracts water.
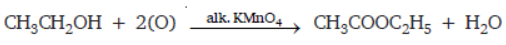
The dirt is carried by hydrophobic portion which is attached to hydrophilic end which gets attached to water and is washed away.
Question. (a) What is homologous series? Give one example.
(b) What will happens if ethanoic acid reacts with ethanol in the presence of an acid as a catalyst? Name the reaction. Write the chemical reaction for this reaction.
(c) Why are soaps ineffective in hard water?
Answer : (a) Homologous series is series of compounds having same functional group and same chemical properties. When members of the series are arranged in ascending order, two successive members differ by –CH2 group and mass 14 u.
Example : Alcohol
CH3OH
CH2 mass 14. a.m.u
C2H5OH
C3H7OH
(b) Ethanol reacts with ethanoic acid to produce a fruity smelling compound called ester, the conc. sulphuric acid is used as dehydrating agent which removes water.
Such reaction is called esterification.
Example:

(c) Hard water contains salts of Ca and Mg. Soap molecule reacts with these salts to produce white precipitate called scum, which is insoluble in water and the cleansing action of soap becomes ineffective.
Question. (a) Distinguish between esterification and saponification reactions of organic compounds.
(b) With a labelled diagram describe an activity to show the formation of an ester.
Answer : (a) Esterification: Reaction in which carboxylic acid reacts with alcohol in presence of conc. H2SO4 to form a fruity smelling compound called ester.
CH3COOH + C2H5OH ⎯ ⎯Con⎯c. H⎯2S⎯O4⎯→CH3COOC2H5 + H2O
Saponification: It is a reaction in which an ester reacts with alkali solution to form a compound called soap.
CH3COOC2H5 + NaOH ⎯⎯→ CH3COONa + C2H5OH
Ester Sodium Sodium Ethanol
hydroxide ethanoate
(b) Activity to show the formation of an ester: Take a test tube and add ethanol, acetic acid and few drops of conc. H2SO4 in it. Warm it over a water bath, i.e., keeping the test tube in a beaker containing water. Pleasant, fruity smelling compound called ester is formed.
CH3COOH + C2H5OH ⎯⎯Con⎯c. H⎯2S⎯O4⎯→ CH3COOC2H5 + H2O (Img 43)
Question. (a) The structural formula of an ester is

Write the structural formula of the corresponding alcohol and the acid.
(b) (i) Mention the experimental conditions involved in obtaining ethene from ethanol.
(ii) Write the chemical equation for the above reaction.
(c) Explain the cleansing action of soap.
Answer : (a) CH3COOH and CH3CH2OH
Ethanoic acid Ethanol
(b) (i) Ethanol when heated in presence of conc. H2SO4, it gets dehydrated to form ethene at 160°C – 170°C.
(c) Cleansing action of soap: Soap consists of two ends, long chain of hydrocarbon is called hydrophobic which repels water and attracts dirt and grease. The other end is hydrophilic which attracts water. (Img 44)
When soap is added to water it forms micelle structure as shown below.
The tail of the soap sticks to the dirt inwards and the head points outward. When water is agitated, the dirt sticks to more number of soap molecules. On lot of rinsing with water, the water washes away soap molecule with dirt attached to it.
Question. A student reports the police about the illegal vending of alcohol near his school. He also knew about denatured alcohol.
(a) What is denatured alcohol?
(b) What would happen if somebody consumes denatured alcohol?
(c) What value is reflected by a student who reported the matter to police?
Answer : (a) Denatured alcohol is ethanol when added with poisonous methanol or copper sulphate solution.
(b) On drinking denatured alcohol a person may die.
(c) Value reflected by student is society’s law and order.
Question. Suman always carried her tiffin box in a jute bag while most of her friends got it packed in a poly bag.
(a) What type of bonding is present in polythene?
(b) Give one advantage of carrying jute and disadvantage of poly bag.
(c) Which value is reflected in Suman by using jute bag?
Answer : (a) In polythene, long chain of ethene is present – C = C –.
(b) Jute bag is biodegradable and will not cause pollution. While poly bag is nonbiodegradable and causes pollution.
(c) Suman shows the value of a responsible behaviour.
Question. Geeta helps her mother in washing clothes, toilets, balconies every Sunday. She uses thele ftover detergent water of washing machine to clean toilets.
(a) Why is detergent used in washing clothes?
(b) Give one advantage of detergent over soap.
(c) What value of Geeta is reflected in the above task?
Answer : (a) Detergents have strong cleansing ability and can remove oil and dirt from clothes or other surfaces.
(b) Soap cannot be used in hard water but detergents can be used in hard water.
(c) Geeta is trying to reduce water pollution and water shortage problem. She also shows helpful to her mother and responsible behaviour.
Question. What are detergents chemically? List two merits and two demerits of using detergents for cleansing. State the reason for the suitability of detergents for washing, even in the case of water having calcium and magnesium ions.
Answer: Detergents chemically are sodium or potassium salts of sulphonic acid of benzene or alkene.
Merits:
(i) They work well with hard water.
(ii) They are more effective than soaps.
Demerits:
(i) They are expensive.
(ii) Some of them having branching are non-biodegradable, therefore create water pollution.
Detergents are suitable for hard water having Mg2+ and Ca2+ ions because they do not form insoluble salts with Mg2+ and Ca2+ ions.
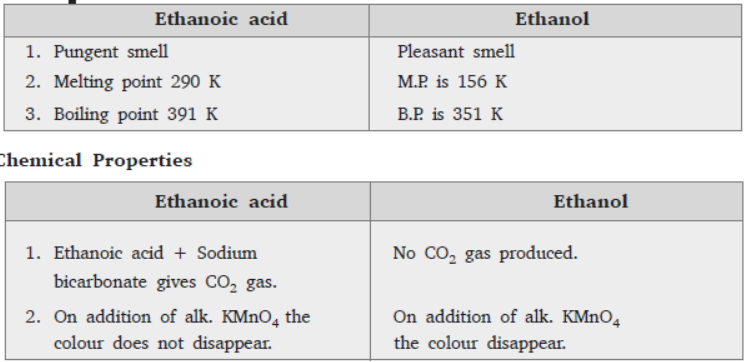
Question. List in tabular form three physical and two chemical properties on the basis of which ethanol and ethanoic acid can be differentiated
Answer:

Question. Explain isomerism. State any four characteristics of isomers. Draw the structures of possible isomers of butane, C4H10
Answer: Isomerism is a phenomenon due to which some compounds have same molecular formula but different structural formulae.
Characteristics:
(i) They differ in structural formula.
(ii) They differ in melting point.
(iii) They differ in boiling point.
(iv) They differ in solubility in same solvent.

Question. Give reasons for the following:
(i) Element carbon forms compounds mainly by covalent bonding.
(ii) Diamond has a high melting point.
(iii) Graphite is a good conductor of electricity.
(iv) Acetylene bums with a sooty flame.
(v) Kerosene does not decolourise bromine water while cooking oils do.
Answer:
(i) It is because carbon has four valence electrons, it cannot gain or lose four electrons because high energy is needed. It can only share four electrons.
(ii) It is due to strong covalent bonds and compact structure of diamond.
(iii) It is due to presence of free electrons in graphite because each carbon is linked to three more carbon atoms.
(iv) It is due to high percentage of carbon, it burns with sooty or smoky flame.
(v) Kerosene oil is mixture of saturated hydrocarbons therefore does not decolourise bromine water.
Question. What is the difference between the chemical composition of soaps and detergents? State in brief the action of soaps in removing an oily spot from a shirt. Why are soaps not considered suitable for washing where water is hard?
Answer: oaps are sodium or potassium salts of fatty acids having — COONa group. Detergents are sodium or potassium salts of sulphonic acids having — SO3Na and — SO4Na group. Cleansing action of soap: Soap molecules consist of a large hydrocarbon tail which is hydrophobic (water-hating or water repelling) with a negatively charged head which is hydrophilic (water-loving) as shown in figure. img
When a soap is dissolved in water, the molecules associate together as clusters called micelles in which water molecules, being polar in nature, surround the ions and the hydrocarbon part of the molecule attracts grease, oil and dirt.
Question. (a) In tabular form, differentiate between ethanol and ethanoic acid under the following heads:
(i) Physical state (ii) Taste
(iii) NaHCO3 test (iV) Ester test
(b) Write a chemical reaction to show the dehydration of ethanol.
Answer:
Question. (a) State two properties of carbon which lead to a very large number of carbon compounds.
(b) Why does micelle formation take place when soap is added to water? Why are micelles not formed when soap is added to ethanol?
Answer:
(a) (i)-Catenation (ii) Tetravalency
(b) It is because large number of molecular ions of soaps get aggregated and form colloidal solution.
Soap has hydrophobic tail (hydrocarbon) which dissolves in hydrocarbon part and hydrophilic part dissolves in water. Ethanol is non-polar solvent therefore micelles are not formed because hydrocarbon part gets attracted towards ethanol and ionic end will not dissolve in alcohol.
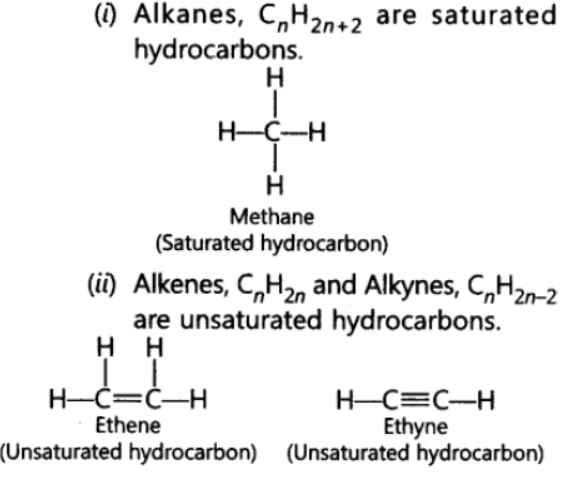
Question. What are the hydrocorbons write the name and general formula of (i) sturated hydrocarbons, (ii) unsaturated hydrocarbons, and draw the structure of one hydrocarbon of each type. How can an unsaturated hydrocarbon be made saturated?
Answer:

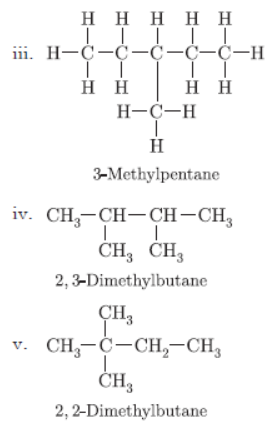
Question. You are given balls and stick model of six carbon atoms and fourteen hydrogen atoms and sufficient number of sticks. In how many ways one can join the models of six carbon atoms and fourteen hydrogen atoms to form different molecules of C6H14.
Answer :
There are five ways in which six carbons can be joined with 14 hydrogen atoms.

Question. Why are certain compounds called hydrocarbons?
Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction which converts alkene into alkane. Also write the chemical equation to show the necessary conditions for the reaction to occur.
Answer : Compounds of carbon and hydrogen are called hydrocarbons.
Alkane CnH2n+2

Question. Complete the following chemical equations and write the chemical name of the products formed.
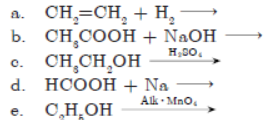
Answer :
Question. a. Give a chemical test to distinguish between saturated and unsaturated hydrocarbons.
b. Name the products formed when ethane burns in air. Write the balanced chemical equation for the reactions showing two types of energies liberated.
c. Why is reaction between methane and chlorine in presence of sunlight is considered a substitution reaction.
Answer :
a. Saturated hydrocarbons will not react with bromine water whereas unsaturated hydrocarbons will decolourise it.
b. Carbon dioxide and water will be formed.
2C2H6 + 7O2 → 4CO2(g) + 6H2O(l)
+ Heat + Light
c. It is because hydrogen atom is substituted by halogen atom, that is why it is called substitution reaction.
Question. a. You have three unlabelled test tubes containing ethanol, ethanoic acid and soap solution. Explain the method you would use to identify the compounds in different test tubes by chemical tests using litmus paper and sodium metal.
b. Give reason of formation of scum when soaps are used with hard water.
Answer : a. Red litmus paper will become blue in soap solution only. Ethanoic acid will turn blue litmus red only. Ethanol will react with Na metal to form sodium nethoxide and hydrogen gas will be liberated.
b. Soaps are sodium or potassium salts of fatty acids which react with Ca2+ and Mg2+ ions in hard water to form calcium or magnesium salts of fatty acids which are insoluble in water called scum.
Question. What are micelles? Why does it form when soap is added to water? Will a micelle be formed in other msolvents such as ethanol also? State briefly how the nformation of micelles help to clean the clothes having oily spots.
Answer : Micelles are cluster of molecules in which hydrophobic tails are inside the cluster 3 and the ionic ends are at the surface of clusters. Soap molecules when dissolved in water they form a cluster due to hydrophobic part of molecules orient themselves away from water.
So they arrange towards inside of the cluster while hydrophilic part remain outside of cluster.
No, micelles will not be formed in alcohol. Soap in form of micelles is able to clean because the oily dirt will be collected in centre of micelle which is rinsed away by water.
Question. a. Differentiate between soap and detergent.
b. Explain why, soaps form scum with water whereas detergent do not.
Answer :
| Soap | Detergent | |
| 1. | Sodium salts of fatty acids. | Sodium salts of sulphonic acids. |
| 2. | They do not work well with hard water. | They work well even with hard water. |
b. Sodium salts of fatty acids (soaps) react with Ca2+ and Mg2+ ions in hard water to form insoluble salts called scum. Detergents form soluble salts with Ca2+ and Mg2+.
Question. List in tabular form three physical and two chemical properties on the basis of which ethanol and ethanoic acid can be differentiated.
Answer :

Question. a. How is vinegar made?
b. What is glacial acetic acid? What is its melting point?
c. Why are carboxylic acids called weak acids?
d. Write the name and formula of compounds formed when the ester CH3COOC2H5 undergoes saponification.
Answer :
a. Vinegar is 5-8% solution of acetic acid (Ethanoic acid) in water. It can be made by fermentation of ethanol in presence of oxygen.
b. Glacial acetic is pure (100%) acetic acid. Its melting point is 290 K.
c. They do not ionise completely in aqueous solution.
d. CH3COOC2H5 + NaOH → CH3COONa + C2H5OH
Sodium ethanoate Ethanol
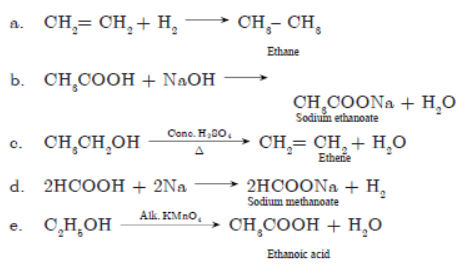
Question. a. How will you bring out following reactions? Write the concerned chemical reaction.
(1) Ethanol to ethene
(2) Ethanol to ethanoic acid
b. Give one example with chemical equation for the following reactions:
(1) Substitution reaction
(2) Saponification reaction
(3) Combustion reaction
Answer :
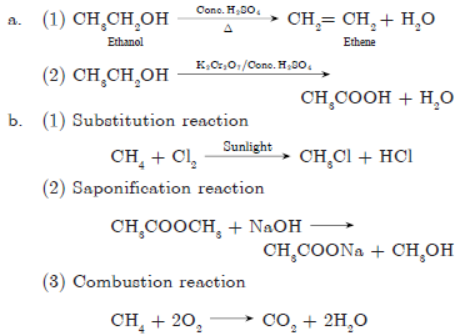
Question. Identify the compounds ‘A’ to ‘E’ in the following sequence:

Answer :
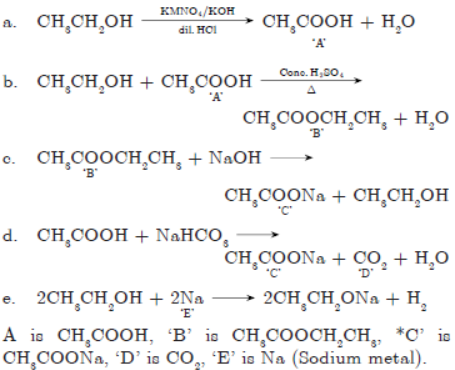
Question. An organic compound “X’ on heating with cone.
H2SO4 forms a compound ‘Y’ which on addition of one molecule of hydrogen in the presence of nickel forms a compound ‘Z’. One molecule of compound ‘Z’ on combustion forms two molecules of CO2 and three molecules of H2O. Identify giving reasons the compounds X’, ‘Y’ and ‘Z’. Write the chemical equations for all the chemical reactions involved.
Answer :
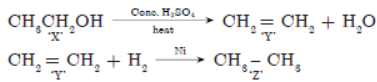
C2H6 + (7/2)O2 → 2CO2 + 3H2O
‘Z’ on combustion gives 2CO2 and 3H2O so hydrocarbon ‘Z’ must be ethane. “Y’ on addition of H2 gives ethane so ‘Y’ must be ethene. “X’ in presence of cone. H2SO4 dehydrates to ethene i.e., ‘X’ is ethanol.
Question. What are detergents chemically? List two merits and two demerits of using detergents for cleansing. State the reason for the suitability of detergents for washing even in case of water having calcium and magnesium ions.
Answer : Detergents are sodium or potassium salts of sulphonic acids of benzene or sulphates of unsaturated hydrocarbons like alkenes with —SO3Na or —SO4Na group.
Merits:
They are more effective than soaps.
They work well even with hard water.
Demerits:
a. They are expensive.
b. Some of them create water pollution.
Question. a. In a tabular form, differentiate between ethanol and ethanoic acid under the following heads:
(i) Physical state
(ii) Taste
(iii) NaHCO3 test
(iv) Ester test
b. Write a chemical reaction to show dehydration of ethanol.
Answer :
(i)
| Ethanol | Ethanoic acid | |
| (a) | Physical taste: It is liquid. | It is solid below 290 K |
| (b) | Taste: It has burning taste. | It has sour taste. |
| (c) | NaHCO3 test It does not react. | It liberates CO2 gas. |
| (d) | Ester test: It reacts with carboxylic acid to form easter | It reacts with alcohol to form pleasant to form fruity smelling esters. |
Question. Soaps and detergents are both types of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps.
Answer :
a. Soaps are sodium or potassium salts of fatty acids e.g. —COONa. Detergents are sodium or potassium salts of sulphonic acids e.g. —SO3Na or —SO4Na
b. Soaps are sodium or potassium salts of fatty acids. They contain —COONa group. Detergents are sodium or potassium salts of sulphonic acids.
They contains —SO3Na or —SO4Na group. Soap has ionic end which is hydrophilic, interacts with water while carbon chain is hydrophobic interacts with oil, grease. The soap molecules orient themselves in a cluster in which hydrophobic tails are inside the cluster and ionic ends face outside.
These cluster are called micelles. These attract oil which is washed away by water.
c. Soaps react with Ca2+ and Mg2+ ions in hard water to form calcium or magnesium salts of fatty acids which are insoluble in water and thus interfere in action of soap,
d. (i) Detergents are more expensive than soaps.
(ii) Some detergents are not biodegradable i.e. will create pollution.
Question. Give reasons for the following:
a. Element carbon forms compound mainly by covalent bonding.
b. Diamond has high melting point.
c. Graphite is good conductor of electricity.
d. Acetylene bums with sooty flame.
e. Kerosene does not decolourise bromine water whereas cooking oil does.
Answer :
a. It is because carbon can neither lose 4 electrons nor gain 4 electrons. It can share four electrons to form covalent bonds.
b. Diamond has strong C—C bonds and compact 3-D structure in which one carbon atom is covalently bonded to other four carbon atoms therefore, has high melting point.
c. In graphite, one carbon atom is bonded to other three carbon atoms. Remaining one electron on each carbon is free to move due to which graphite conducts electricity.
d. Acetylene has high carbon content, therefore, partial oxidation causes it to bum with sooty or smoky flame.
e. Kerosene is a saturated compound, therefore, does not decolourise bromine water.


