Please refer to the Class 11 Chemistry Sample Paper below. These CBSE Sample Papers for Class 11 Chemistry have been prepared based on the latest guidelines and examination patterns issued for the current academic year. We have provided Term 1 and Term 2 sample papers with solutions. You can click on the links below and access the free latest CBSE Sample Papers for Chemistry for Standard 11. All guess papers for Chemistry Class 11 have been prepared by expert teachers. You will be able to understand the type of questions which are expected to come in the examinations and get better marks.
CBSE Sample Papers for Class 11 Chemistry
| Term 2 Sample Papers |
| Class 11 Chemistry Sample Paper Term 2 With Solutions Set A |
Class 11 Chemistry Sample Paper Term 2 With Solutions Set A
SECTION – A
1. Read the passage given below and answer the following questions.
The salts of alkali metals are ionic and soluble in water. The solubility of an ionic compound depends on
two factors :(i) Lattice energy and (ii) Hydration energy. These two factors oppose each other. If hydration energy is high, the ions will have greater tendency to be hydrated and therefore the solubility with be high. The smaller the cation, the greater is the degree of its hydration. Hydration of ions is an exothermic process. Higher is the energy released if greater is the degree of hydration. Reducing power of alkali metals in solutions is also dependent on the hydration energy beside other factors.
(i) The radius of which ion (hydrated) is lowest?
(a) [Li(aq)]+
(b) [Na(aq)]+
(c) [K(aq)]+
(d) [Cs(aq)]+
Answer
D
(ii) The ion which has maximum value of hydration energy is
(a) Li+
(b) Na+
(c) Cs+
(d) K+
Answer
A
OR
Ionic mobility of Li+ is less than Na+ and K+ ions in solutions because
(a) ionisation potential of lithium is small
(b) charge density of Li+ is high
(c) high hydration tendency of Li+
(d) Li+ keeps two electrons.
Answer
C
(iii) Strongest reducing agent amongst alkali metals in solutions is
(a) Li
(b) Na
(c) K
(d) Cs
Answer
A
(iv) Which has least solubility?
(a) CsF
(b) LiF
(c) NaF
(d) KF
Answer
B
Following questions (Q. No. 2 – 6) are multiple choice questions carrying 1 mark each:
2. Which of the following statements is incorrect?
(a) B(OH)3 partially reacts with water to form H3O+ and [B(OH)4]–, and behaves like a weak acid.
(b) B(OH)3 behaves like a strong monobasic acid in the presence of sugars, and this acid can be titrated against an NaOH solution using phenolphthalein as an indicator.
(c) B(OH)3 does not donate a proton and hence does not form any salt with NaOH.
(d) B(OH)3 reacts with NaOH, forming Na[B(OH)4].
Answer
C
3. An alkene on ozonolysis gives methanal as one of the product. Its structure is

Answer
C
OR
Which of the following reactions is not an example of electrophilic substitution in benzene ring?
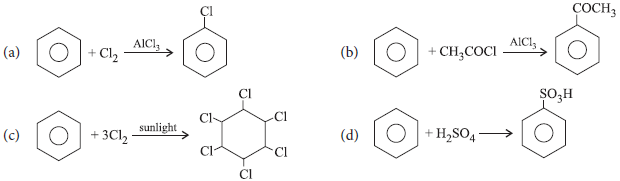
Answer
C
4. Classify the following as acid or base according to Bronsted – Lowry concept.

Answer
C
5. Graphs between pressure and volume are plotted at different temperatures. Which of the following isotherms represents Boyle’s law as PV = constant?

(a) Only (ii) is correct representation of Boyle’s law.
(b) Only (iv) is correct representation of Boyle’s law.
(c) All are correct representations of Boyle’s law.
(d) None of these representations is correct for Boyle’s law.
Answer
C
OR
Molecular mass of a gas is 78. Its density at 98°C and 1 atm will be
(a) 200 g L–1
(b) 2.56 g L–1
(c) 256 g L–1
(d) 78 g L–1
Answer
B
6. The concentration of Ag+ ion in a saturated solution of Ag2CrO4 at 20°C is 1.5 × 10–4 mol L–1. The solubility product of Ag2CrO4 at 20°C is
(a) 1.687 × 10–12
(b) 1.75 × 10–10
(c) 3.0 × 10–8
(d) 4.5 × 10–10
Answer
A
In the following questions (Q. No. 7 and 8) a statement of assertion following by a statement of reason is given.Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is the correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not the correct explanation of assertion.
(c) Assertion is correct statements but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
7. Assertion : Lithium resembles magnesium diagonally placed in next group.
Reason : The size of Li+ and Mg2+ are different and their electronegative character is same.
Answer
C
OR
Assertion : Alkali metals are obtained by electrolysis of molten salt and not aqueous solution.
Reason : The discharge potential of H+ ions is lower than alkali metal cation hence hydrogen is discharged at cathode instead of metal.
Answer
A
8. Assertion: Wurtz reaction is not preferred for the preparation of alkanes containing odd number of carbon atoms.
Reason : It is not possible to prepare alkanes with odd number of carbon atoms through Wurtz reaction.
Answer
C
SECTION – B
The following questions, Q. No. 9-12 are short answer type and carry 2 marks each.
9. (a) Diamond is very hard while graphite is soft. Why?
(b) CO2 is a gas while SiO2 is a solid. Why?
Answer : (a) Diamond has giant three dimensional polymeric structure in which each carbon is sp3 hybridized and linked to four carbon atoms. This structure makes diamond hardest. On account of small radii of carbon atoms, the various atoms are closely packed in the crystal lattice. Graphite possesses layer structure in which each carbon atom is sp2 hybridized. There is wide separation between various layers. One layer can slide easily on the other. This makes graphite soft in nature.
(b) In carbon dioxide discrete molecules are present. In each molecule carbon is linked with two oxygen atoms by double bonds (OCO), while silica possesses a giant three dimensional structure in which each silicon is linked with four oxygen atoms tetrahedrally and each oxygen is linked with two silicon atoms.

This structure is extremely stable and considerable energy is required to break SiO bond. Thus, CO2 is a gas and silica is a solid.
OR
(a) Why aluminium chloride forms a dimer?
(b) Lead pollution is caused by car exhaust. Explain.
Answer : (a) Aluminium atom in aluminium chloride contains 6 electrons in its outermost orbit. So it requires 2 more electrons for completion of its octet. This is fulfilled by dimerisation when chlorine atoms donate lone pairs to aluminium atoms as shown below:

(b) The petrol contains antiknock agent, tetra ethyl lead [(C2H5)4Pb] which burns with petrol containing a little C2H5Br which forms volatile PbBr2 and this PbBr2 comes out with car exhaust.
10. Account for the formation of both 3-bromo-2,2-dimethylbutane and 2-bromo-2,3-dimethylbutane from the reaction of HBr with 3,3-dimethyl-1-butene.
Answer : Reaction occurs as:

Conclusion: Along with the expected alkyl bromide an isomer is also formed due to rearrangement.
11. At 3000 K, the equilibrium pressures of CO2, CO and O2 are 0.6, 0.4 and 0.2 atm respectively.
(a) Calculate Kp for the reaction : 2CO2 ⇌ 2CO + O2.
(b) Show how Kp is related to Kc.
(c) Calculate Kc (R = 0.082 L atm K–1 mol–1).
Answer : (a) Applying law of mass action :

12. If 1 kcal of heat is added to 1.2 L of oxygen in a cylinder at constant pressure of 1 atm, the volume increases to 1.5 L. Calculate ΔE for the process.
Answer :

OR
Calculate the minimum work which must be done to compress 1/2 mole of oxygen at 300 K from a pressure of 2 atm to a pressure of 200 atm.
Answer :

SECTION – C
Q. No. 13 and 14 are short answer type II carrying 3 marks each.
13. An aqueous solution of a gas (X) shows the following reactions:
(a) It turns red litmus blue
(b) When added in excess to copper sulphate solution, a deep blue colour is obtained.
(c) On addition of FeCl3 solution a brown precipitate, soluble in dilute HNO3 is obtained. Identify (X) and give reactions.
Answer : The aqueous solution of gas is basic hence, it should be ammonia (NH3).
Aqueous ammonia in excess forms a deep blue, soluble complex with copper sulphate solution.
With FeCl3 solution, aqueous ammonia gives a brownish precipitate of Fe(OH)3 which dissolves in dilute HNO3.
Reactions:
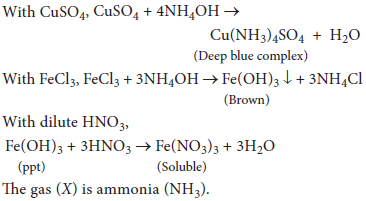
14. (a) Using the data given below, calculate the value of equilibrium constant for the reaction at 298 K
3HC ≡ CH(g) ⇌ C6H6(g)
Acetylene Benzene
assuming ideal gas behaviour.
ΔG°f (HC ≡ CH(g)) = 2.09 × 105 J mol–1
ΔG°f (C6H6(g)) = 1.24 × 105 J mol–1
R = 8.314 J K–1 mol–1
(b) Based on your calculated value, comment whether this process can be recommended as a practical method for making benzene.
Answer :
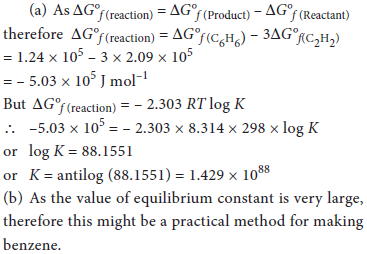
OR
For the reaction, 2A(g) + B(g) → 2C(g), calculate ΔG° and predict whether the reaction may occur spontaneously, ΔE° = –2.50 kcal and ΔS° = –10.5 cal K–1.
Answer :
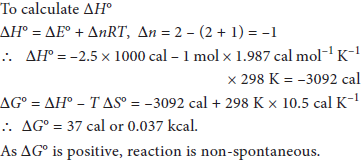
SECTION – D
Q. No. 15 and 16 are long answer type carrying 5 marks each.
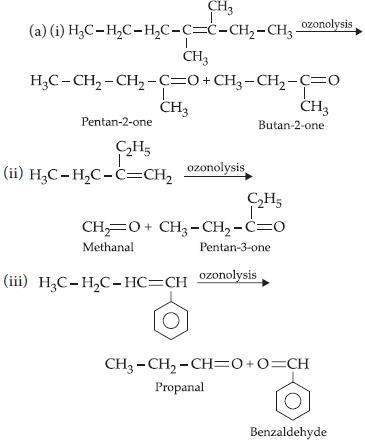
15. (a) Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) 3,4-dimethylhept-3-ene
(ii) 2-ethylbut-1-ene
(iii) 1-phenylbut-1-ene
(b) An alkene A on ozonolysis gives a mixture of ethanal and pentan-3-one. Write structure and IUPAC name of A.
Answer :
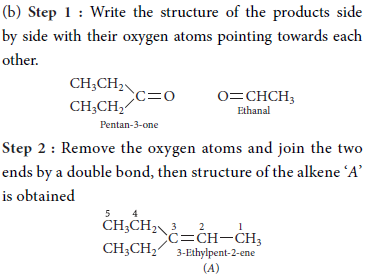
OR
(a) Explain the following:
(i) Benzene is highly unsaturated but undergoes substitution reactions easily rather than addition.
(ii) All m-directors are deactivating.
(iii) Halogens, as exceptions, are o- and p-directors but are deactivating.
(iv) Most o- and p-directors are activating.
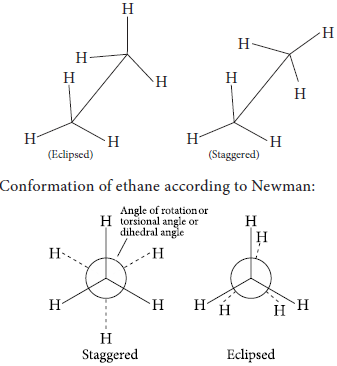
(b) Draw Newman and Sawhorse projections of ethane molecule (eclipsed form).
Answer : (a) (i) Benzene molecule is stabilised by resonance (delocalisation of p-electrons) and as substitution does not disturb this stabilisation, it occurs easily. Addition reactions, on the other hand disturb the resonance stabilisation or aromatic character, thus they are resisted.
(ii) The meta directing substituents withdraw electrons from the benzene ring and thus, deactivate it for further substitution.
(iii) If a halogen substituent is present, two opposing effects operate simultaneously. Halogen atom releases electrons due to resonance but withdraws electrons due to its high electronegativity (–I effect). The –I effect is greater than resonance effect, therefore the benzene ring is deactivated.
(iv) The o- and p-directing substituents release electrons to the benzene ring thereby activating it for further substitution.
(b) Conformations of ethane using sawhorse projection:
16. (a) When 2 g of a gas A is introduced into an evacuated flask kept at 25°C, the pressure is found to be 1 atm. If 3 g of another gas B is then added to the same flask, the total pressure becomes 1.5 atm. Assuming ideal gas behavior, calculate the ratio of molar masses MA and MB.
(b) A spherical balloon of 21 cm diameter is to be filled up with hydrogen at STP from a cylinder containing the gas at 20 atm at 27°C. If the cylinder can hold 2.82 lit. of water. Calculate the number of balloons that can be filled up.
Answer :
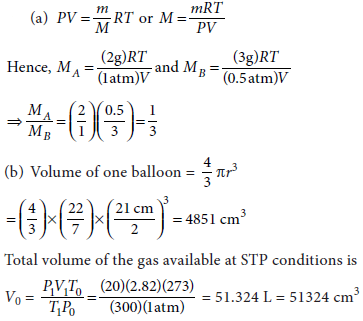
When the balloons are being filled, the pressure in the cylinder will decrease. We can continue filling the cylinder till the pressure within the cylinder is also 1 atm. At this stage the volume of 2820 cm3 of the gas will remain within the cylinder.

OR
(a) What will be the pressure of the gaseous mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in a 1 L vessel at 27°C?
(b) A 20 g chunk of dry ice is placed in an empty 0.75 litre wine bottle tightly closed. What would be the final pressure in the bottle after all CO2 has been evaporated and temperature reaches to 25°C?
Answer : Partial pressure of hydrogen gas
V1 = 0.5 L V2 = 1.0 L
P1 = 0.8 bar P2 = ?
Applying Boyle’s law (constant T and n)





