Please refer to Class 12 Chemistry Sample Paper With Solutions Set D provided below. The Sample Papers for Class 12 Chemistry have been prepared based on the latest pattern issued by CBSE. Students should practice these guess papers for class 12 Chemistry to gain more practice and get better marks in examinations. The Sample Papers for Chemistry Standard 12 will help you to understand the type of questions which can be asked in upcoming examinations.
Sample Paper for Class 12 Chemistry With Solutions Set D
1. 0.6 mole of PCl5, 0.3 mole of PCl3 and 0.5 mole of Cl2 are taken in a 1 L flask to obtain the following equilibrium:
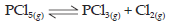
If the equilibrium constant Kc for the reaction is 0.2 predict the direction of the reaction.
(a) Forward direction
(b) Backward direction
(c) Direction of the reaction cannot be predicted.
(d) Reaction does not move in any direction.
Answer
B
2. Which of the following orders about the basic strengths is correct?
(a) NH3 < NH2OH < HN3 < NH2NH2
(b) NH2OH < HN3 < NH2NH2 < NH3
(c) HN3 < NH3< NH2OH < NH2NH2
(d) HN3 < NH2OH < NH2NH2 < NH3
Answer
B
3. In Bohr series of lines of hydrogen spectrum, the third line from the red end corresponds to which one of the following inter-orbit jumps of the electron for Bohr orbits in an atom of hydrogen?
(a) 3 → 2
(b) 5 → 2
(c) 4 → 1
(d) 2 → 5
Answer
B
4. Which of the following enzymes is not useful in the digestion of proteins?
(a) Chymotrypsin
(b) Pepsin
(c) Trypsin
(d) Lipase
Answer
D
5. Which of the following conformations is optically inactive?
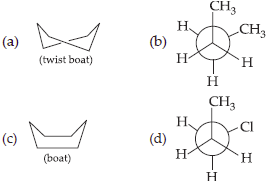
Answer
C
6. Which of the following statements is not true?
(a) XeO3 is an explosive compound.
(b) Noble gases neither burn nor help in burning.
(c) Two S – O bonds in SO2 molecule are not equal.
(d) The electron affinity of noble gases is zero.
Answer
C
7. Which of the following compounds will exhibit cis-trans (geometrical) isomerism?
(a) 2-Butene
(b) 2-Butyne
(c) 2-Butanol
(d) Butanal
Answer
A
8. The correct order of decreasing thermalstability of the hydrides of group 16 elements is
(a) H2S > H2Te > H2Po > H2O > H2Se
(b) H2Se > H2S > H2Po > H2O > H2Te
(c) H2O > H2Se > H2Te > H2S > H2Po
(d) H2O > H2S > H2Se > H2Te > H2Po
Answer
D
9. The time for half life period of a certain reaction A Products is 1 hour. When the initial concentration of the reactant A → is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1 if it is a zero order reaction?
(a) 1 h
(b) 4 h
(c) 0.5 h
(d) 0.25 h
Answer
D
10. An ether is more volatile than an alcohol having the same molecular formula. This is due to
(a) dipolar character of ethers
(b) alcohols having resonance structures
(c) intermolecular hydrogen bonding in ethers
(d) intermolecular hydrogen bonding in alcohols.
Answer
D
11. Which of the following does not show stereoisomerism?
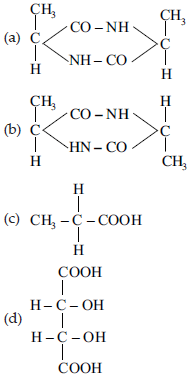
Answer
C
12. An organic compound of molecular formula C3H6O does not produce any precipitate with 2,3-dinitrophenylhydrazine and does not react with sodium metal. This compound is
(a) CH3COCH3
(b) CH2 = CH – OCH3
(c) CH3CH2CHO
(d) CH2 = CHCH2OH
Answer
B
13. The probability of finding out an electron at a point within an atom is proportional to the
(a) square of the orbital wave function i.e., ψ2
(b) orbital wave function i.e., ψ
(c) Hamiltonian operator i.e., H
(d) principal quantum number i.e., n
Answer
A
14. What is the correct order of acidity from weakest to strongest acid for these compounds?
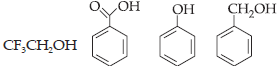
(a) I < IV < III < II
(b) III < IV < I < II
(c) IV < I < III < II
(d) II < III < I < IV
Answer
C
15. Which of the following does not give oxygen on heating?
(a) K2Cr2O7
(b) (NH4)2Cr2O7
(c) KClO3
(d) Zn(ClO3)2
Answer
B
16. Which of the following will produce a buffer solution when mixed in equal volumes?
(a) 0.1 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
(b) 0.05 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
(c) 0.1 mol dm–3 NH4OH and 0.05 mol dm–3 HCl
(d) 0.1 mol dm–3 CH3COONa and 0.1 mol dm–3 NaOH
Answer
C
17.
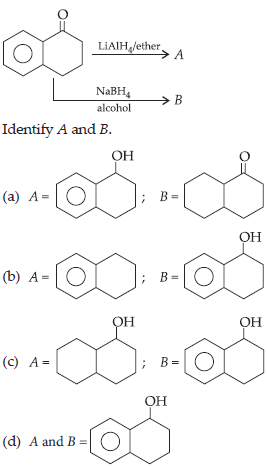
Answer
D
18. The emf of the three galvanic cells are represented by E1, E2 and E3.
I. Zn | Zn2+ (1 M) || Cu2+ (1 M) | Cu
II. Zn | Zn2+ (0.1 M) || Cu2+ (1 M) | Cu
III. Zn | Zn2+ (1 M) || Cu2+ (0.1 M) | Cu
Which of the following is true?
(a) E1 > E2 > E3
(b) E3 > E2 > E1
(c) E3 > E1 > E2
(d) E2 > E1 > E3
Answer
D
19. Non-stoichiometric metal deficiency is shown in the salts of
(a) all metals
(b) alkali metals only
(c) alkaline earth metals only
(d) transition metals only.
Answer
D
20. The product of acid hydrolysis of P and Q can be distinguished by
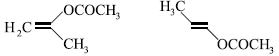
(a) Lucas reagent
(b) 2,4-DNP
(c) Fehling’s solution
(d) NaHSO3
Answer
C
21. Interstitial compounds are
(a) non-stoichiometric and are ionic in nature
(b) non-stoichiometric and are covalent in nature
(c) non-stoichiometric and are neither typically ionic nor covalent
(d) stoichiometric and are neither ionic nor covalent.
Answer
C
22. Calorific value is in the order
(a) fats > carbohydrates > proteins
(b) carbohydrates > fats > proteins
(c) proteins > carbohydrates > fats
(d) fats > proteins > carbohydrates.
Answer
A
23. CsCl crystallises in body-centred cubic lattice. If ‘a’ is its edge length then which of the following expressions is correct?
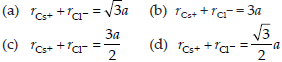
Answer
D
24. In which of the following reactions, Kp > Kc ?
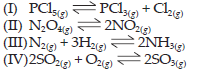
(a) (III) and (IV)
(b) (II) and (III)
(c) (I) and (II)
(d) (I), (II) and (IV)
Answer
C
25. Which of the following orders of relative strengths of acids is correct?
(a) FCH2COOH > ClCH2COOH > BrCH2COOH
(b) ClCH2COOH > BrCH2COOH > FCH2COOH
(c) BrCH2COOH > ClCH2COOH > FCH2COOH
(d) ClCH2COOH > FCH2COOH > BrCH2COOH
Answer
A
26. Which of the following changes will increase the emf of the cell?
Co(s) | CoCl2(M1) || HCl(M2) | H2(g) | Pt(s)
(a) Increase the volume of the CoCl2 solution from 100 to 200 mL
(b) Increase M2 from 0.010 to 0.500 M
(c) Increase the pressure of H2(g) from 1.00 to 2.00 atm
(d) Increase the mass of the Co-electrode from 10.0 to 20.0 g
Answer
B
27. The magnitude of CFSE (Crystal Field Splitting Energy, Δo) can be related to the configuration of d-orbitals in a coordination entity as

Answer
A
28. In an antifluorite structure, cations occupy
(a) octahedral voids
(b) centre of cube
(c) tetrahedral voids
(d) corners of cube.
Answer
C
29. Which one of the following is an example of Hell-Volhard-Zelinsky reaction?
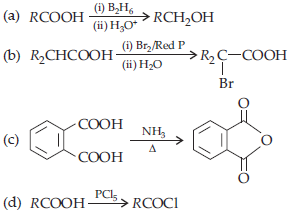
Answer
B
30. A radiation of wavelength λ illuminates a metal and ejects photoelectrons of maximum kinetic energy of 1 eV. Another radiation of wavelength λ/3 , ejects photoelectrons of maximum kinetic energy of 4 eV. What will be the work function of metal?
(a) 4 eV
(b) 2.5 eV
(c) 0.5 eV
(d) 1.5 eV
Answer
C
31. Ketones do not reduce Tollens’ reagent, but fructose with a keto group reduces it. It is attributed to
(a) enolisation of keto group of fructose and then, its transformation into aldehyde group in presence of OH– which is present in Tollens’ reagent
(b) CHOH group which is also oxidised to keto group
(c) both statements are correct
(d) none of these.
Answer
A
32. Assuming that water vapour is an ideal gas, the internal energy change (ΔU) when 1 mol of water is vapourised at 1 bar pressure and 100°C, (given : molar enthalpy of vapourisation of water at 1 bar and 373 K = 41 kJ mol–1 and R = 8.3 J mol–1 K–1) will be
(a) 41.00 kJ mol–1
(b) 4.100 kJ mol–1
(c) 3.7904 kJ mol–1
(d) 37.904 kJ mol–1
Answer
D
33. White phosphorus on reaction with NaOH gives PH3 as one of the products. This is a
(a) dimerization reaction
(b) disproportionation reaction
(c) condensation reaction
(d) precipitation reaction.
Answer
B
34. The solubility product of AgI at 25°C is 1.0 × 10–16 mol2 L–2. The solubility of AgI in 10–4 N solution of KI at 25°C is approximately (in mol L–1)
(a) 1.0 × 10–8
(b) 1.0 × 10–16
(c) 1.0 × 10–12
(d) 1.0 × 10–10
Answer
C
35. What is the decreasing order of basicity of primary, secondary and tertiary ethylamines and NH3?
(a) NH3 > C2H5NH2 > (C2H5)2NH > (C2H5)3N
(b) (C2H5)3N > (C2H5)2NH > C2H5NH2 > NH3
(c) (C2H5)2NH > C2H5NH2 > (C2H5)3N > NH3
(d) (C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3
Answer
D
36. The unit cell of aluminium is a cube with an edge length of 405 pm. The density of aluminium is 2.70 g cm–3. What is the structure of unit cell of aluminium?
(a) Body-centred cubic cell
(b) Face-centred cubic cell
(c) End-centred cubic cell
(d) Simple cubic cell
Answer
B
37. The most appropriate set of reagents for carrying out the following conversion is
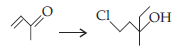
(a) EtMgBr ; HCl
(b) (C2H5)2CuLi ; HCl
(c) C2H5Li ; HCl
(d) HCl ; EtMgBr
Answer
D
38. In the nitration of benzene with a mixture of concentrated nitric acid and concentrated sulphuric acid, the active species involved is
(a) nitrite ion
(b) nitrate ion
(c) nitronium ion
(d) nitric oxide.
Answer
C
39. Activation energy (Ea) and rate constants (k1 and k2) of a chemical reaction at two different temperatures (T1 and T2) are related by
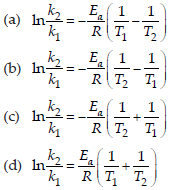
Answer
B
40. Low spin complex of d6 cation in an octahedral field will have the following energy
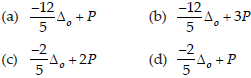
(Δo = Crystal field splitting energy in an octahedral field, P = Electron pairing energy)
Answer
B
