Students can refer to the following Chemical Reactions and Equations MCQ Questions for Class 10 with Answers provided below based on the latest curriculum and examination pattern issued by CBSE and NCERT. Our teachers have provided here a collection of multiple choice questions for Chemical Reactions and Equations Class 10 covering all topics in your textbook so that students can assess themselves on all important topics and thoroughly prepare for their exams
Chemical Reactions and Equations MCQ Questions for Class 10 with Answers
We have provided below Chemical Reactions and Equations MCQ Questions for Class 10 with answers which will help the students to go through the entire syllabus and practice multiple choice questions provided here with solutions. As Chemical Reactions and Equations MCQs for Class 10 Science pdf download can be really scoring for students, you should go through all problems provided below so that you are able to get more marks in your exams.
Chemical Reactions and Equations MCQ Questions for Class 10
Question: On the basis of following features, identify the correct option.
(i) This reaction occurs during corrosion.
(ii) This reaction occurs during respiration.
(a) Decomposition reaction
(b) Redox reaction
(c) Combination reaction
(d) Endothermic reaction
Answer
B
Question: Which of the following is not a physical change?
(a) Boiling of water to give water vapour.
(b) Melting of ice to give water.
(c) Dissolution of salt in water.
(d) Combustion of Liquefied Petroleum Gas (LPG).
Answer
D
Question: Which of the following is a decomposition reaction?
(a) 2HgO Heat→2Hg + O2
(b) CaCO3 Heat→CaO + CO2
(c) 2H2O Electrolysi →H2 + O2
(d) All of these
Answer
D
Question: In which of the following the identity of initial substance remains unchanged?
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food
Answer
B
Question: When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of –
(a) a combination reaction
(b) a displacement reaction
(c) a decomposition reaction
(d) a double decomposition reaction
Answer
D
Question. Fe2O3 + 2Al ⎯⎯→ Al2O3 + 2Fe
The above reaction is an example of a:
(a) combination reaction
(b) double displacement reaction
(c) decomposition reaction
(d) displacement reaction
Answer
D
Question. Which of the statements about the reaction below are incorrect?
2PbO (s) + C (s) ⎯⎯→ 2Pb (s) + CO2 (g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting reduced.
(c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) All
Answer
A
Question. What happens when dilute hydrochloric acid is added to iron filings? Tick the correct answer:
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Answer
A
Question: Which of the following reactions involves the combination of two elements?
(a) CaO + CO2 → CaCO3
(b) 4Na + O2 → 2Na2O
(c) SO2 + 1/2 O2→ SO3
(d) NH3 + HCl → NH4Cl
Answer
B
Question: Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
Answer
C
Question: What happens when copper rod is dipped in iron sulphate solution?
(a) Copper displaces iron
(b) Blue colour of copper sulphate solution is obtained
(c) No reaction takes place
(d) Reaction is exothermic
Answer
C
Question: A dilute solution of sodium carbonate was added to two test tubes (A) containing dil HCl and (B) containing dilute NaOH. The correct observation was –
(a) a brown coloured gas liberated in test tube A.
(b) a brown coloured gas liberated in test tube B.
(c) a colourless gas liberated in test tube A.
(d) a colourless gas liberated in test tube B.
Answer
C
Question: A balanced chemical equation is in accordance with –
(a) Avogadro’s law
(b) law of multiple proportion
(c) law of conservation of mass
(d) law of gaseous volumes.
Answer
C
Question: The schematic diagram is given below
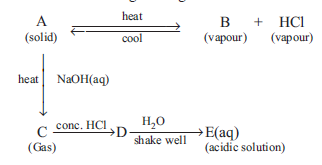
Which of the following is a correct statement ?
(a) A and E are chemically same.
(b) A and D are chemically same.
(c) D and E are chemically same.
(d) C and E are chemically same.
Answer
B
Question: A student added dilute HCl to a test tube containing zinc granules and made following observations which one is correct?
(a) The zinc surface became dull and black.
(b) A gas evolved which burns with a pop sound.
(c) The solution remained colourless.
(d) The solution becomes green in colour.
Answer
B
Question: The reaction in which two compounds exchange their ions to form two new compounds is –
(a) a displacement reaction
(b) a decomposition reaction
(c) an isomerization reaction
(d) a double displacement reaction
Answer
D
Question: When the gases sulphur dioxide and hydrogen sulphide mix in the presence of water, the reaction is SO2 + 2H2S → 2H2O + 2S. Here hydrogen sulphide is acting as –
(a) an oxidising agent
(b) a reducing agent
(c) a dehydrating agent
(d) a catalyst
Answer
B
Question: Zn+ H2SO4(dil) → ZnSO4 + H2↑ Above reaction is –
(a) decomposition reaction
(b) single displacement reaction
(c) combination reaction
(d) synthesis reaction
Answer
B
Question: CuO + H2 → H2O + Cu, reaction is an example of –
(a) redox reaction
(b) synthesis reaction
(c) neutralisation
(d) analysis reaction
Answer
A
Question. Which of the following is a displacement reaction?
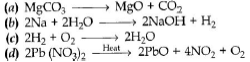
Answer
B
Question.. Magnesium ribbon is rubbed before burning because it has a coating of
(a) basic magnesium carbonate
(b) basic magnesium oxide
(c) basic magnesium sulphide
(d) basic magnesium chloride
Answer
B
Question.. Which of the following statements about the given reaction are correct?
3Fe (s) + 4H2O (g) → Fe3O4 (s) + 4 H2 (g)
(i) Iron metal is getting oxidized
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidizing agent
(a) (i), (ii) and (iii)
(b) (ii) and (iv)
(c) (i), (ii) and (iv)
(d) (ii) and (iv)
Answer
C
Question.. Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
A
Question.. Oxidation is a process which involves
(i) addition of oxygen
(ii) addition of hydrogen
(iii) removal of oxygen
(iv) removal of hydrogen
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
C
Question. Which of the following are chemical changes ?
(i) Digestion of food
(ii) Liquefaction of air
(iii) Ripening of fruit
(iv) Dissolution of sulphur in carbon disulphide
(v) Freezing of water
(vi) Electrolysis of water
(1) (i) to (iv) all
(2) (i), (iii) & (vi)
(3) (i), (iii) & (iv)
(4) (iii), (iv) & (vi)
Answer
2
Question. Which among the following is not a physical change?
(1) Melting of solid to liquid.
(2) Vaporisation of liquids to gas.
(3) Liquefaction of gases to liquid.
(4) Decay of matter.
Answer
4
Question. An example of a chemical change is _____.
(1) formation of clouds.
(2) glowing of an electric light.
(3) dropping sodium into water.
(4) dissolving of salt in water.
Answer
3
Question. Physical changes are usually _______ in nature.
(1) temporary
(2) permanent
(3) irreversible
(4) endothermic
Answer
1
Question. Which among the following is not a chemical change?
(1) Melting of ice.
(2) Carbon cycle.
(3) Dehydration of substances.
(4) Fermentation of substances.
Answer
1
Question. Decomposition of water is
(1) electrolytic
(2) thermal
(3) photolytic
(4) All of the above
Answer
1
Question. Which of the following is a combustion reaction ?
(1) Boiling of water
(2) Melting of wax
(3) Burning of petrol
(4) None of these
Answer
4
Question. The reaction, H2 + Cl2 →2HCl is
(1) an oxidation reaction
(2) a reduction reaction
(3) a combination reaction
(4) an isomerisation reaction
Answer
3
Question. Which of the following is a decomposition reaction ?
(1) NaOH + HCl → NaCl + H2O
(2) NH4CNO → H2NCONH2
(3) 2KClO3 → 2KCl + 3O2
(4) H2 + I2 → 2Hl
Answer
3
Question. Which of the following is not a decomposition reaction ?
(1) CaCO3 → CaO + CO2
(2) 2KClO3 →2KCl + 3O2
(3) Digestion of food in body
(4) H2 + Cl2 →2HCl
Answer
4
Question. Which of these will cause a chemical change to occur ?
(1) Grinding of wheat into flour.
(2) Lighting of a gas stove.
(3) Evaporation of water from a lake.
(4) Ringing of an electric bell.
Answer
2
Question. Chemical changes are __________.
(1) temporary, reversible and a new substance is produced.
(2) always accompanied by exchange of light.
(3) permanent, irreversible and a new substance is produced.
(4) never accompanied by exchange of light and heat energy.
Answer
3
Question. Lead nitrate on heating gives
(1) Lead oxide
(2) Nitrogen dioxide
(3) Oxygen
(4) All of these
Answer
4
Question. Heating limestone produces
(1) Quick lime
(2) Carbon dioxide
(3) Both (1) and (2)
(4) None of these
Answer
3
Question. In the equation Cu + xHNO3 → Cu(NO3)2 + yNO2 + 2H2O The values of x and y are
(1) 3 and 5
(2) 8 and 6
(3) 4 and 2
(4) 7 and 1
Answer
3
Question. Which of the following chemical equations is an unbalanced one ?
(1) 2NaHCO3 → Na2CO3 + H2O + CO2
(2) 2C4H10 + 12O2 → + 8CO2 + 10H2O
(3) 2Al + 6H2O →2Al(OH)3 + 3H2
(4) 4NH3 + 5O2 → 4NO + 6H2O
Answer
2
Question. Which of the following is a physical change?
(1) Solubility in water
(2) Combustibility
(3) Aerial oxidation
(4) Reaction with water
Answer
1
Question. Formation of carbon disulphide from carbon and sulphur takes place by
(1) absorption of heat
(2) evolution of heat
(3) no change in heat content
(4) None of the above
Answer
1
Question. In the reaction given below, a, b, c and d respectively are aFe2O3 + bH2 →cFe + dH2O
(1) 1, 1, 2, 3
(2) 1, 1, 1, 1
(3) 1, 3, 2, 3
(4) 1, 2, 2, 3
Answer
3
Question. When ferrous hydroxide reacts with hydrochloric acid, _______ and H2O are produced.
(1) FeCl3
(2) FeCl2
(3) FeCl4
(4) FeCl
Answer
4
Question. When copper powder is heated, it gets coated with
(1) Black copper oxide
(2) Yellow copper oxide
(3) Red copper oxide
(4) None of these
Answer
1
Question. Which of the following stands for a double displacement reaction ?
(1) 2H2 + O2 → 2H2O
(2) 2Mg + O2 →2MgO
(3) AgNO3 + NaCl → AgCl + NaNO3
(4) H2 + Cl2 → 2HCl
Answer
3
Question. Barium chloride on reacting with ammoniums ulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved ?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction.
(iv) Double displacement reaction
(1) (i) only
(2) (ii) only
(3) (iv) only
(4) (ii) and (iv)
Answer
4
Question. Which of the following equations is representing combination of two compounds ?
(1) CaO + CO2¾→ CaCO3
(2) CO +1/2O2 ¾→ CO2
(3) SO2 +1/2O2 ¾→ SO3
(4) 2Na + 2H2O ¾→ 2NaOH + H2
Answer
1
Question. Wh ich of the followin g is not a t hermal decomposition reaction ?
(1) 2H2O →2H2 + O2
(2) 2FeSO4 → Fe2O3 + SO2 + SO3
(3) ZnCO3 →ZnO + CO2
(4) 2KClO3 → 2KCl + 3O2
Answer
1
Question. Which of the statements about the reaction below are incorrect ?
2PbO(s) + C(s) →2Pb(s) + CO2(g)
(i) Lead is getting reduced.
(ii) Carbon dioxide is getting oxidised.
(iii) Carbon is getting oxidised.
(iv) Lead oxide is getting reduced.
(1) (i) and (ii)
(2) (i) and (iii)
(3) (i), (ii) and (iii)
(4) All of the above
Answer
1
Question. Which of the following is a displacement reaction ?
(1) CaCO3 → CaO + CO2
(2) CaO + 2HCl →CaCl2 + H2O
(3) Fe + CuSO4 → FeSO4 + Cu
(4) NaOH + HCl →NaCl + H2O
Answer
3
Question. What happens when dilute hydrochloric acid is added to iron filings ? Tick the correct answer.
(1) Hydrogen gas and iron chloride are produced.
(2) Chlorine gas and iron hydroxide are produced.
(3) No reaction takes place.
(4) Iron salt and water are produced.
Answer
1
Question. The reaction, Fe2O3(s) + 2Al(s) →Al2O3 + 2Fe(l) is an example of
(1) combination reaction
(2) double displacement
(3) decomposition reaction
(4) single displacement reaction
Answer
4
Question.When green coloured ferrous sulphate crystals are heated, the colour of the crystal changes because:
(a) it is decomposed to ferric oxide
(b) it loses water of crystallisation
(c) it forms SO2
(d) it forms SO3
Answer
B
Question.What is observed when a solution of potassium iodide is added to silver nitrate solution?
(a) No reaction takes place
(b) White precipitate of silver iodide is formed
(c) yellow precipitate of Agl is formed
(d) Agl is soluble in water.
Answer
C
Question. The chemical formula for slaked lime is
(a) Ca(OH)2
(b) CaCO3
(c) CaO
(d) CaHCO3
Answer
A
Question. The chemical formula of lead nitrate is:
(a) KNO3
(b) PbNO3
(c) K(NO3)2
(d) Pb(NO3)2
Answer
D
Question.PbS reacts with ozone (O3) and forms pbso4 . As per the balanced equation, molecules of ozone required for every one molecule of PbS is/are
(a) 4
(b) 3
(c) 2
(d) 1
Answer
A
Question.The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called:
(a) Antioxidation
(b) Reduction
(c) Rancidity
(d) Corrosion
Answer
C
Question.The reaction between lead nitrate and potassium iodide present in aqueous solutions is an example of visit for Topper’s materials
(a) Decomposition Reaction
(b) Displacement Reaction
(c) Double Displacement Reaction
(d) Neutralisation Reaction
Answer
C
Question . An iron nail was dipped in a salt solution. After sometime a reddish brown deposition of the nail was seen. The salt solution could be
(a) Silver nitrate
(b) Sodium sulphate
(c) Aluminium chloride
(d) Copper sulphate
Answer
D
Question. The colour of the residue left in the test tube after heating ferrous sulphate which undergoes decomposition is
(a) yellowish-brown
(b) black
(c) white
(d) grey
Answer
A
Question.Chemically rust is
(a) Hydrated ferrous oxide
(b) hydrated ferric oxide
(c) only ferric oxide
(d) none of these
Answer
B
Question. Which of the following reactions will not take place?
(a) Zn + CuSO4→ ZnSO3+ Cu
(b) 2KBr + Cl2→KCI+ Br2
(c) Zn + MgSO4→ ZnSO4+ Mg
(d) Mg + FeSO4– MgSO4+ Fe
Answer
(c) Zn + MgSO4→ ZnSO4+ Mg
Answer
C
Question.Which of the following is a thermal decomposition reaction?
(a) 2H2O → 2H2+ O2
(b) 2AgCl → 2Ag + Cl2
(c) H2(g) + Cl2(g) → 2HCl(g)
(d) ZnCO3→ ZnO + CO2
Answer
D
Question. Reaction between calcium oxide and water is a ______________ reaction.
(a) endothermic reaction
(b) decomposition reaction
(c) exothermic reaction
(d) displacement reaction
Answer
C
Question. The compound also known as blue vitriol is
(a) FeSO4.7H2O
(b) CuSO4.5H2O
(c) CaSO4.2H2O
(d) Na2CO3.10H2O
Answer
B
Question. The chemical formula for lime is
(a) Ca(OH)2
(b) CaCO3
(c) CaO
(d) CaHCO3
Answer
C
Question. The reaction of water and quick lime is an example of
(a) combination reaction
(b) exothermic reaction
(c) both (a) and (b)
(d) None of these.
Answer
C
Question. How many water molecules are present in a crystal of copper sulphate molecule?
(a) 5
(b) 7
(c) 2
(d) 3
Answer
A
Question. How many water molecules are present in a crystal of ferrous sulphate molecule?
(a) 5
(b) 2
(c) 7
(d) 10
Answer
C
Question. Which of the following is an endothermic reaction?
(a) CaCO3 heat CaO + CO2
(b) CaO + H2O Ca(OH)2
(c) C6H12O6 + O2 6CO2 + 6H2O
(d) None of these
Answer
A
Question: In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(&) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Answer
B
Question: Select the oxidising agent for the following reaction:
H2S + I2 > 2HI + S
(a) I2
(b) H2S
(C) HI
(d) S
Answer
A
Question: A substance added to food containing fats and oils is called:
(a) Oxidant
(b) Rancid
(c) Coolant
(d) Antioxidant
Answer
D
Question: Which among the following statement(s) is (are) true? Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride
(ii) sublimation of silver chloride
(iii decomposition of chlorine gas from silver chloride
(iv) oxidation of silver chloride
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iv) only
Answer
A
Question: The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called:
(a) antioxidation
(b) reduction
(c) rancidity
(d) corrosion
Answer
C
Question: Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
(a) 1 : 1
(b) 2:1
(c) 4:1
(d) 1:2
Answer
B
Question: What type of chemical reactions take place when electricity is passed through water?
(a) Displacement
(b) Combination
(c) Decomposition
(d) Double displacement
Answer/ Explanation
Answer
C
Question: When SO2 gas is passed through saturated solution of H2S, which of the following reaction occurs?
(a) SO2 + 2H2S → 2H2O + 3S
(b) SO2 + 2H2S → H2O + 3S
(c) SO2 + H2S → H2O + S
(d) SO2 + H2O → SO3 + H2
Answer
A
Question: Pb + CuCl2 → PbCl2 + Cu
The above reaction is an example of:
(a) combination
(b) double displacement
(c) decomposition
(d) displacement
Answer
D
Question: Which of the following gases can be used for storage
(a) Carbon dioxide or Oxygen
(b) Nitrogen or Oxygen
(c) Carbon dioxide or Helium
(d) Helium or Nitrogen
Answer
D
Question:Name the products formed when iron filings are heated with dilute hydrochloric acid
(a) Fe (III) chloride and water
(b) Fe (II) chloride and water
(c) Fe (II) chloride and hydrogen gas
(d) Fe (III) chloride and hydrogen gas
Answer
D
Question. What is the colour of FeSO4.7H2O?
(a) Blue
(b) Green
(c) White
(d) Brown
Answer
B
Assertion-Reason Questions
In the following question, a statement of Assertion
(A) is followed by a statement of Reason (R). Answer these questions by selecting appropriate option given below:
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
(b) Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of Assertion (A)
(c) Assertion (A) is true but Reason (R) is false.
(d) Assertion (A) is false but Reason (R) is true.
Question. Assertion (A): Pungent smelling gas is produced when sulphur burns in air.
Reason (R): Sulphur trioxide is formed on reaction of sulphur with oxygen.
Answer
C
Question. Assertion (A): To dilute sulphuric acid, acid is added to water and not water to acid.
Reason (R): Specific heat of water is quite large.
Answer
A
Question. Assertion (A): Calcium Carbonate when heated gives calcium oxide and water.
Reason (R): On heating CaCO3, decomposition reaction takes place.
Answer
D
Question. Assertion (A): Sodium metal is stored under Kerosene.
Reason (R): Metallic sodium melts when exposed to air.
Answer
C
Question. Assertion (A): A reducing agent is a substance which can either accept electron.
Reason (R): A substance which helps in oxidation is known as reducing agent.
Answer
D
Question. Assertion (A): Quicklime reacts vigorously with water releasing a large amount of heat.
Reason (R): The above chemical reaction is an exothermic reaction.
Answer
A
Question. Assertion (A): Photosynthesis is considered as an endothermic reaction.
Reason (R): Energy gets released in the process of photosynthesis
Answer
C
Question. Assertion (A): Fe2O3 + 2Al → Al2O3 + 2Fe The above chemical equation is an example of displacement reaction.
Reason (R): Aluminium being more reactive than iron, displaces Fe from its oxide.
Answer
A
Question. Assertion (A): Stannous chloride is a powerful oxidising agent which oxidises mercuric chloride to mercury.
Reason (R): Stannous chloride gives grey precipitate with mercuric chloride, but stannic chloride does not do so.
Answer
C
Question. Assertion (A): Carbon dioxide turns lime water milky.
Reason (R): Carbon dioxide sullies the water.
Answer
C
Assertion-Reason Questions
(a) Both ‘A’ and ‘R’ are true and ‘R’ is correct explanation of the assertion.
(b) Both ‘A’ and ‘R’ are true but ‘R’ is not correct explanation of the assertion.
(c) ‘A’ is true but ‘R’ is false.
(d) ‘A’ is false and ‘R’ is true.
Question. Assertion: CO + 2H2 340 atm→ Δ CH3OH(l)
Reason: It is combination reaction because CO combines with H2 to form CH3OH i.e. two substances combine to form a single compound.
Answer
B
Question. Assertion: A. In electrolysis of water volume of hydrogen is twice the volume of oxygen.
Reason: H2 gas is liberated at cathode and O2 at anode.
Answer
B
Question. Assertion: A reducing agent is a substance which can accept electron.
Reason: A substance which helps in oxidation is known as oxidising agent.
Answer
D
Question. Assertion: White silver chloride turns grey in sunlight.
Reason: Decomposition of silver chloride in presence of sunlight takes place to form silver metal and chlorine gas.
Answer
A
Question. Assertion: In the following chemical equation, CuO(s) + Zn(s) → ZnO(s) + Cu(s) Zinc is getting oxidised and copper oxide is getting reduced.
Reason: The process in which oxygen is added to a substance is called oxidation whereas the process in which oxygen is removed from a substance is called reduction.
Answer
A
Question. Assertion: All combustion reactions are exothermic.
Reason: Heat is absorbed in endothermic reactions.
Answer
B
Question. Assertion: The reaction, MnO2 + 4HCl → MnCl2 + 2H2O + Cl2 is an example of redox reaction.
Reason: In this reaction, HCl is reduced to Cl2 whereas MnO2 is oxidized to MnCl2.
Answer
C
Assertion-Reason Questions
(a) Both ‘A’ and ‘R’ are true and ‘R’ is correct explanation of the assertion.
(b) Both ‘A’ and ‘R’ are true but ‘R’ is not correct explanation of the assertion.
(c) ‘A’ is true but ‘R’ is false.
(d) ‘A’ is false and ‘R’ is true.
Question. Assertion: Iron articles get rusted in moist air.
Reason: Moisture and oxygen required for rusting to form hydrated ferric oxide.
Answer
A
Question. Assertion: Chips and other snacks become rancid due to oxidation.
Reason: To prevent the food like chips and other snacks they are packed in carbon dioxide gas.
Answer
C
Very Short Answer Type Questions
Question. Which one is a chemical change-rusting of iron or melting of iron?
Answer. Rusting of iron.
Question. Name and state the law which is kept in mind while we balance a chemical equation.
Answer. Law of conservation of mass. Mass can neither be created nor be destroyed during a chemical reaction.
Question. State one basic difference between a physical change and a chemical change.
Answer. In a physical change, no new substance is formed. In a chemical change, a new substance is formed.
Question. What happens when quicklime is added to water?
Answer. Quicklime reacts with water vigorously to produce slaked lime and a large amount of heat.
Question. What happens when ZnCO3 is heated in the absence of air? Give the relevant equation.
Answer. ZnO(s) and CO2(g)CO2(g) are formed. Chemical Equation:
ZnCO3 → ZnO + CO2
Case Study Based Questions
Read the following and answer any four questions from (i) to (v):
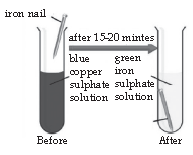
In the below experiment, when an iron nail is dipped in copper sulphate solution, a brown coating of copper is formed in the surface of iron and the colour of copper sulphate solution changes from blue to pale green. The reaction shows that iron is more reactive than copper because it displaces copper from the copper sulphate solution.
Question. In the following equation:
Cu + xHNO3 → Cu(NO3)2 + yNO2 + 2H2O.
The values of x and y are:
(a) 3 and 5
(b) 8 and 6
(c) 4 and 2
(d) 7 and 1
Answer
C
Question. When copper rod is dipped in iron sulphate solution:
(a) Copper displaces iron
(b) Blue colour of copper sulphate solution is obtained
(c) No reaction takes place
(d) Reaction is exothermic
Answer
C
Question. A substance which oxidised itself and reduces other is known as:
(a) Oxidising agent
(b) Reducing agent
(c) Both (a) and (b) ‘
(d) None of these
Answer
B
Question. Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:
(a) Combination reaction
(b) Double displacement reaction
(c) Decomposition reaction
(d) Displacement reaction
Answer
D
Question. Name the products formed when iron filings are heated with dilute hydrochloric acid.
(a) Fe (III) chloride and water
(b) Fe (II) chloride and water
(c) Fe (II) chloride and hydrogen gas
(d) Fe (III) chloride and hydrogen gas
Answer
C