Check the below NCERT Aldehydes Ketones and Carboxylic Acids MCQ Questions for Class 12 with Answers available with PDF free download. MCQ Questions for Class 12 Chemistry with Answers were prepared based on the latest syllabus and examination pattern issued by CBSE, NCERT and KVS. Our teachers have provided below Aldehydes Ketones and Carboxylic Acids Class 12 Chemistry MCQs Questions with answers which will help students to revise and get more marks in exams
Aldehydes Ketones and Carboxylic Acids MCQ Questions for Class 12 Chemistry with Answers
Refer below for Aldehydes Ketones and Carboxylic Acids MCQ Questions for Class 12 Chemistry with solutions. Solve questions and compare with the answers provided below
Question.Which one of the following can be oxidised to the corresponding carbonyl compound?
(a) 2-Hydroxypropan
(b) ortho-Nitrophenol
(c) Phenol
(d) 2-Methyl-2-hydroxypropane
Answer
A
Question. In the reaction, CH3CN + 2H → HCl Ether → X→ Boiling H2OY; the term Y is
(a) acetaldehyde
(b) ethanamine
(c) acetone
(d) dimethylamine.
Answer
A
Question. Ketones [RCOR1] where R = R1 = alkyl group. It can be obtained in one step by
(a) oxidation of tertiary alcohol
(b) reaction of acid halide with alcohols
(c) hydrolysis of esters
(d) oxidation of primary alcohol.
Answer
A
Question. The oxidation of toluene to benzaldehyde by chromyl chloride is called
(a) Etard reaction
(b) Riemer–Tiemann reaction
(c) Wurtz reaction
(d) Cannizzaro’s reaction.
Answer
A
Question. Reaction between benzaldehyde and acetophenone in presence of dilute NaOH is known as
(a) Aldol condensation
(b) Cannizzaro’s reaction
(c) Cross Cannizzaro’s reaction
(d) Cross Aldol condensation.
Answer
D
Question. Which of the following reagents would distinguish cis-cyclopenta-1,2-diol from the trans-isomer?
(a) MnO2
(b) Aluminium isopropoxide
(c) Acetone
(d) Ozone
Answer
C
Question. The correct statement regarding a carbonyl compound with a hydrogen atom on its alphacarbon, is
(a) a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as carbonylation
(b) a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as keto-enol tautomerism
(c) a carbonyl compound with a hydrogen atom on its alpha-carbon never equilibrates with its corresponding enol
(d) a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as aldehyde-ketone equilibration
Answer
B
Question. The product formed by the reaction of an aldehyde with a primary amine is
(a) carboxylic acid
(b) aromatic acid
(c) Schiff ’s base
(d) ketone.
Answer
C
Question. Reaction of a carbonyl compound with one of the following reagents involves nucleophilic addition followed by elimination of water. The reagent is
(a) hydrazine in presence of feebly acidic solution
(b) hydrocyanic acid
(c) sodium hydrogen sulphite
(d) a Grignard reagent.
Answer
A
Question. CH3CHO and C6H5CH2CHO can be distinguished chemically by
(a) Benedict’s test
(b) iodoform test
(c) Tollens’ reagent test
(d) Fehling’s solution test.
Answer
B
Question. Consider the reaction : RCHO + NH2NH2 → RCH=N — NH2 What sort of reaction is it?
(a) Electrophilic addition-elimination reaction
(b) Free radical addition-elimination reaction
(c) Electrophilic substitution-elimination reaction
(d) Nucleophilic addition-elimination reaction
Answer
D
Question. Which of the following compounds will give a myellow precipitate with iodine and alkali?
(a) Acetophenone
(b) Methyl acetate
(c) Acetamide
(d) 2-Hydroxypropane
Answer
A,D
Question. Clemmensen reduction of a ketone is carried out in the presence of which of the following?
(a) Glycol with KOH
(b) Zn-Hg with HCl
(c) LiAlH4
(d) H2 and Pt as catalyst
Answer
B
Question. Which of the following reactions will not result in the formation of carbon-carbon bonds?
(a) Reimer–Tiemann reaction
(b) Cannizzaro reaction
(c) Wurtz reaction
(d) Friedel–Crafts acylation
Answer
B
Question. A strong base can abstract an a-hydrogen from
(a) ketone
(b) alkane
(c) alkene
(d) amine.
Answer
A
Question. Reduction of aldehydes and ketones into hydrocarbons using zinc amalgam and conc. HCl is called
(a) Cope reduction
(b) Dow reduction
(c) Wolff–Kishner reduction
(d) Clemmensen reduction.
Answer
D
Question. The product formed in aldol condensation is A
(a) a beta-hydroxy aldehyde or a beta-hydroxy ketone
(b) an alpha-hydroxy aldehyde or ketone
(c) an alpha, beta unsaturated ester
(d) a beta-hydroxy acid.
Answer
A
Question. Which of the following can be used to differentiate between aldehyde and ketone?
(a) Ammoniacal AgNO3
(b) Ammoniacal AgNO3 in the presence oftartarate ion
(c) I2 in the presence of base
(d) Ammoniacal AgNO3 in the presence of citrate ion
Answer
A
Question. A compound X undergoes reduction with LiAIH4 to yield Y . When vapours of Yare passed over freshly reduced copper at 300° C, X is formed. What is Y?
(a) CH3COCH3
(b) CH3CHO
(c) CH3CH2OH
(d) CH3OCH3
Answer
C
Question. The product formed in the aldol condensation of acetaldehyde is
(a) CH3CH2CH(OH)CHO
(b) CH3CH(OH)CH2CHO
(c) CH3CH(OH)COCH3
(d) CH3CH2CH2CHO
Answer
B
Question. The reaction of an organic compound with ammonia followed by nitration of the product gives a powerful explosive, called RDX. The organic compound is
(a) phenol
(b) toluene
(c) glycerine
(d) formaldehyde
Answer
D
Question. Which of the following does not give Fehling solution test?
(a) Acetone
(b) Propanal
(c) Ethanal
(d) Butanal
Answer
A
Question. Aldo! condensation is given by
(a) trimethyl acetaldehyde
(b) acetaldehyde
(c) benzaldehyde
(d) formaldehyde
Answer
B
Question. Which of the following will form two isomers with semi carbazide ?
(a) Benzaldehyde
(b) Acetone
(c) Benzoquinone
(d) Benzophenone
Answer
A
Question. In the reaction
CH3COOH →LiAIH4 A →PCl5 B →Ale.KOH C, the product C is
(a) acetaldehyde
(b) acetylene
(c) ethylene
(d) acetyl chloride
Answer
C
Question. Identify Din the following reaction
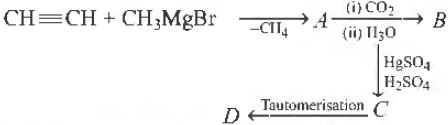
(a) HOOC—CH2—COOH
(b) OHC—CH2—COOH
(c) OHC—CH2—CHO
(d) HO—CH=CH—COOH
Answer
B
Question. A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was
(a) CH3OH
(b) HCHO
(c) CH3COCH3
(d) CH3COOC2H5
Answer
D
Question. 2-pentanone and 3-petanone can be distinguished by
(a) Cannizzaro’s reaction
(c) Iodoform reaction reduction
(b) Aldo! condensation
(d) Clemmensen’s
Answer
C
Question. Which of the product is formed when acetone is reacted with barium hydroxide solution?

Answer
A
Question. The correct increasing order of the acid strength ofbenzoic acid (I), 4-nitrobenzoic acid (II), 3, 4-dinitrobenzoic acid (III) and 4methoxybenzoic acid (IV) is
(a) I < II < III < IV
(b) II < I < IV < III
(c) IV < I < II < III
(d) IV < II < I < III
(e) I < IV < II < III
Answer
C
Question. Vinegar is dilute aqueous solution of
(a) ethanoic acid
(b) benzoic acid
(c) citric acid
(d) oxalic acid
Answer
A
Question. Which one of the following acid does not have a carboxylic group?
(a) Methanoic acid
(b) Ethanoic acid
(c) Propanoic acid
(d) Picric acid
Answer
D
Question. Sodium phenoxide when heated with CO2 under pressure at 125°C yields a product which on acetylation producesC.
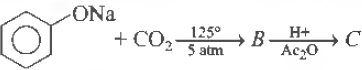

Answer
A
Question. The compound that undergoes decarboxyliation most readily under mild condition is
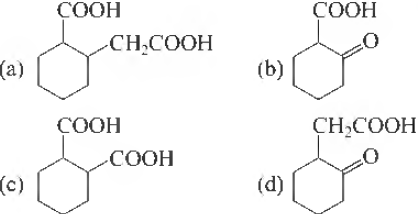
Answer
B
Question. Among the following compounds, the one(s) that gives (give) effervescence with aqueous NaHCO3 solution is (are)
(CH3CO)2 OCH3COOHPhOHCH3COCHO
I II III IV
(a) I and II
(b) I and III
(c) Only II
(d) 1 and IV
Answer
A
Question. Which acid is present in vinegar?
(a) Formic acid
(b) Acetic acid
(c) Butyric acid
(d) Tartaric acid
Answer
B
Question. What product is formed when cyclohexanone is oxidised?
(a) HOOC. (CH2)4 · COOH
(b) CH3CH2COOH
(c) CH3(CH2)4COOH
(d) None of the above
Answer
A
Question. Identify the method by which Me3CCO2H can be prepared.
(a) Treating 1 mole of MeCO Me with 2 moles of MeMgI
(b) Treating 1 mole of MeCO2 Me with 3 moles of MeMgI
(c) Treating 1 mole of MeCHO with 3 moles of MeMgI
(d) Treating 1 mole of dry ice with 1 mole of Me3CMgI
Answer
D
Question.
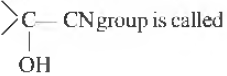
(a) hydroxy nitrite
(c) cyanohydrin
(b) hydroxy cyanide
(d) hydroxy isocyanide
Answer
C
Question. Benzaldehyde on refluxing with aqueous alcoholic KCN produce
(a) cyanobenzene
(b) cyanohydrin
(c) benzoyl cyanide
(d) benzoin
Answer
D
Question. Which one of the following is an Hell-Volhard-Zelinsky reaction?

Answer
B
Question. The strongest acid amongst the following compounds is
(a) CH3COOH
(b) HCOOH
(c) CH3CH2CH(Cl)CO2H
(d) ClCH2CH2CH2COOH
Answer
C
Question. A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of a-hydroxy acid. The carbonyl compound is
(a) formaldehyde
(b) acetaldehyde
(c) acetone
(d) diethyl ketone.
Answer
B
Question. In this reaction : CH3CHO + HCN → CH3CH(OH)CN →HOH CH3CH(OH)COOH an asymmetric centre is generated. The acid obtained would be
(a) D-isome
(b) L-isomer
(c) 50% D + 50% L-isomer
(d) 20% D + 80% L-isomer.
Answer
C
Question. Which of the following is incorrect?
(a) FeCl3 is used in detection of phenol.
(b) Fehling solution is used in detection of glucose.
(c) Tollens’ reagent is used in detection of unsaturation.
(d) NaHSO3 is used in detection of carbonyl compound.
Answer
C
Question. Polarisation in acrolein can be described as
(a) +δCH2=CH — +δCHO
(b) -δCH2= CH — +δCHO
(c) -δCH2= CH — CHO+δ
(d) +δCH= CH — CHO-δ
Answer
D
Question. First product of the reaction between RCHO and NH2NH2 is
(a) RCH NNH2
(b) RCH NH
(c) RCH2NH2
(d) RCON3
Answer
A
Question. An ester (A) with molecular formula, C9H10O2 was treated with excess of CH3MgBr and the complex so formed, was treated with H2SO4 to give an olefin (B). Ozonolysis of (B) ave a ketone with molecular formula C8H8O which shows +ve iodoform test. The structure of (A) is
(a) H3CCH2COC6H5
(b) C2H5COOC6H5
(c) C6H5COOC2H5
(d) p-H3CO – C6H4 – COCH3
Answer
C
Question. Iodoform test is not given by
(a) ethanal
(b) ethanol
(c) 2-pentanone
(d) 3-pentanone.
Answer
D
Question. Phenylmethanol can be prepared by reducing the benzaldehyde with
(a) CH3Br and Na
(b) CH3I and Mg
(c) CH3Br
(d) Zn and HCl.
Question. The oxidation of toluene with CrO3 in the presence of (CH3CO)2O gives a product A, which on treatment with aqueous NaOH produces
(a) C6H5COONa
(b) 2, 4-diacetyl toluene
(c) C6H5CHO
(d) (C6H5CO)2O
Answer
A
Question. When aniline reacts with oil of bitter almonds (C6H5CHO) condensation takes place and benzal derivative is formed. This is known as
(a) Schiff ’s base
(b) Benedict’s reagent
(c) Millon’s base
(d) Schiff ’s reagent.
Answer
A
Question. Compound A has a molecular formula C2Cl3OH. It reduces Fehling’s solution and on oxidation, it gives a monocarboxylic acid B. If A is obtained by the action of chlorine on ethyl lcohol, then compound A is
(a) methyl chloride
(b) monochloroacetic acid
(c) chloral
(d) chloroform.
Answer
C
Question. Which of the following compounds will undergo self aldol condensation in the presence of cold dilute alkali?
(a) CH C — CHO
(b) CH2 CHCHO
(c) C6H5CHO
(d) CH3CH2CHO
Answer
D
Question. Which of the following compounds will give positive test with Tollens’ reagent?
(a) Acetic acid
(b) Acetone
(c) Acetamide
(d) Acetaldehyde
Answer
D
Question. (CH3)2C= CHCOCH3 can be oxidised to (CH3)2C=CHCOOH by
(a) chromic acid
(b) NaOI
(c) Cu at 300°C
(d) KMnO4
Answer
B
Question. In which of the following, the number of carbon atoms does not remain same when carboxylic acid is obtained by oxidation?
(a) CH3COCH3
(b) CCl3CH2CHO
(c) CH3CH2CH2OH
(d) CH3CH2CHO
Answer
A
Question. Acetaldehyde reacts with
(a) electrophiles only
(b) nucleophiles only
(c) free radicals only
(d) both electrophiles and nucleophiles.
Answer
B
Question. The reagent which can be used to distinguish acetophenone from benzophenone is
(a) 2,4-dinitrophenylhydrazine
(b) aqueous solution of NaHSO3
(c) Benedict reagent
(d) I2 and NaOH.
Answer
D
Question. If formaldehyde and KOH are heated, then we get
(a) methane
(b) methyl alcohol
(c) ethyl formate
(d) acetylene.
Answer
B
Question. Which of the following compounds gives benzoic acid on hydrolysis?
(a) Chlorobenzene
(b) Benzoyl chloride
(c) Chlorophenol
(d) Chlorotoluene
Answer
B
Question. Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
(a) formation of intramolecular H-bonding
(b) formation of carboxylate ion
(c) more extensive association of carboxylic acid via van der Waals’ forces of attraction
(d) formation of intermolecular H-bonding.
Answer
D
Question. The correct order of decreasing acid strength of trichloroacetic acid (A), trifluoroacetic acid (B), acetic acid (C) and formic acid (D) is
(a) B > A > D > C
(b) B > D > C > A
(c) A > B > C > D
(d) A > C > B > D
Answer
A
Question. An organic compound A on treatment with NH3 gives B, which on heating gives C. C when treated with Br2 in the presence of KOH produces ethyl amine. Compound A is
(a) CH3COOH
(b) CH3CH2CH2COOH
(c) CH3— CHCOOH ι
CH3
(d) CH3CH2COOH
Answer
D
Question. Which of the following represents the correct order of the acidity in the given compounds?
(a) FCH2COOH > CH3COOH > BrCH2COOH > ClCH2COOH
(b) BrCH2COOH > ClCH2COOH > FCH2COOH > CH3COOH
(c) FCH2COOH > ClCH2COOH > BrCH2COOH > CH3COOH
(d) CH3COOH > BrCH2COOH > ClCH2COOH > FCH2COOH
Answer
C

We hope you liked MCQ Class 12 Chemistry Chapter 12 Aldehydes Ketones and Carboxylic Acids with answers provided above. Incase you have any questions please post them in the comments section below and our Chemistry teachers will provide a response.
