Please refer to Class 10 Science Sample Paper Term 1 With Solutions Set C provided below. The Sample Papers for Class 10 Science have been prepared based on the latest pattern issued by CBSE. Students should practice these guess papers for class 10 Science to gain more practice and get better marks in examinations. The Term 1 Sample Papers for Science Standard 10 will help you to understand the type of questions which can be asked in upcoming examinations.
Term 1 Sample Paper for Class 10 Science With Solutions Set C
Question. Arrange the following in the increasing order of pH values.
A. NaOH solution
B. Blood
C. Lemon juice
D. Milk of magnesia
(a) C < B < D < A
(b) A < B < C < D
(c) D < C < B < A
(d) A < B < D < C
Answer
A
Question. Which of the following are correctly matched?
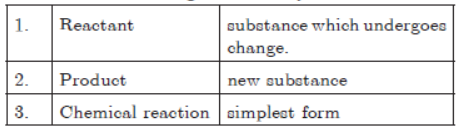
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
A
Question. The image formed by a concave mirror is observed to be virtual, erect and larger than the object. Where should be the position of the object?
(a) Between the principal focus and the centre of curvature
(b) At the centre of curvature
(c) Beyond the centre of curvature
(d) Between the pole of the mirror and its principal focus.
Answer
D
Question. Ethane (C2H6) on complete combustion gave CO2 and water. It shows that the results are in accordance with the law of conservation of mass. Then, the coefficient of oxygen is equal to
(a) 7/2
(b) 3/2
(c) 5/2
(d) 9/2
Answer
A
Question. A light ray enters from medium A to medium B as shown in figure. The refractive index of medium A relative to B will be

(a) greater than unity
(b) less than unity
(c) equal to unity
(d) zero
Answer
A
Question. Identify the micro-organism whose nutrition type is shown below :

(a) Food bacteria
(b) Yeast
(c) Fungus
(d) Amoeba
Answer
D
Question. A student takes about 6 ml of distilled water in each of the four test tubes A, B, C and D, then dissolves in equal amount four different salts name sodium chloride in A Potassium Chloride in B, Calcium Chloride in C and magnesium chloride in D. He then adds 10 drop of soap solution to each test tube and shakes its contents. The test tube(s) in which he would observe a good amount of lather is:
(a) A and B
(b) Only A
(c) C and D
(d) Only B
Answer
A
Question. The process by which all the by products discarded from the body is known as:
(a) Respiration
(b) Sweating
(c) Excretion
(d) None of the above
Answer
C
Question. A body wanted to remove the grease strain from our shirt. So he used a X solution. Here X solution is:
(a) Ammonium hydroxide
(b) Magnesium hydroxide
(c) Calcium hydroxide
(d) Sodium hydroxide
Answer
A
Question. Which of the following is carried by lymph which is digested and absorbed from intestine?
(a) Fat
(b) Protein
(c) Minerals
(d) Carbohydrates
Answer
A
Question. Which of the following are used as an antacid to reduce acidity in stomach?
(a) Sodium carbonate and magnesium hydroxide
(b) Magnesium hydroxide and sodium hydroxide
(c) Sodium bicarbonate and calcium hydroxide
(d) Sodium bicarbonate and magnesium hydroxide
Answer
D
Question. FeS H2SO4 FeSO4 H2S + $ + -. In the above equation – indicates:
(a) gas evolved
(b) insoluble substance formed
(c) reactive element
(d) element is not useful in chemical equation
Answer
A
Question. Which one of the following is not a function of kidney?
(a) Filtration
(b) Oxidation
(c) Absorption
(d) Secretion
Answer
B
Question. ………. helps in trans location of food in plants.
(a) Xylem
(b) Phloem
(c) Palisade cells
(d) Root hairs
Answer
B
Question. An object 5.0 cm in length is placed at a distance of 20 cm in front of a convex mirror or radius of curvature 30 cm. The position of the image is-
(a) 8.57 cm
(b) 9.10 cm
(c) 8.15 cm
(d) 7.15 cm
Answer
A
Question. Which of the following substances will not give carbon dioxide on treatment with dilute acid?
(a) Marble
(b) Limestone
(c) Baking soda
(d) Lime
Answer
D
Question. Sunita takes about 2 g ferrous sulphate crystals in dry boiling tube and heat the boiling tube over the flame of a burner or spirit lamp as shown in the figure. 36 The colour of crystals after heating is:

(a) Black
(b) Brown
(c) Green
(d) Orange
Answer
B
Question. The radius of curvature of a spherical mirror is 20 cm. the focal length of mirror is
(a) 10 cm
(b) 20 cm
(c) 30 cm
(d) 40 cm
Answer
A
Question. An element X (atomic number 12) reacts with another element Y (atomic number 17) to form a compound Z . Which of the following statements are true regarding this compound?
1. Molecular formula of Z is XY2.
2. It is soluble in water.
3. X and Y are joined by sharing of electrons.
4. It would conduct electricity in the molten state.
(a) 2 and 3
(b) 1 and 2
(c) 1, 3 and 4
(d) 1, 2 and 4
Answer
D
Question. Which one of the following is NOT present in urine?
(a) Water
(b) Salts
(c) Urea
(d) Salivary amylase
Answer
D
Question. Which of the following cannot be beaten into thin sheets?
(a) Sulphur
(b) Aluminium
(c) Iron
(d) Zinc
Answer
A
Question. Light enters from air to glass having refractive index 1.50. The speed of light in vacuum is 3#108 ms−1. The speed of light in the glass is-
(a) 2#108ms−1
(b) 3#108ms−1
(c) 4#104ms−1
(d) 5#105ms−1
Answer
A
Question. Mohit arranged two metal rods in electrolyte solution as shown in the figure and electron flows from metal X to metal Y

(a) Copper, Zinc
(b) Zinc, Silver
(c) Iron, Aluminium
(d) Iron, Silver
Answer
B
Question. A full length image of a distant tall building can definitely be seen by using
(a) a concave mirror
(b) a convex mirror
(c) a plane mirror
(d) both concave as well as plane mirror
Answer
B
Question. A concave mirror produces three times magnified (enlarged) real image of an object placed at 10 cm in front of it. Where is the image located?
(a) 30 cm
(b) 40 cm
(c) −30 cm
(d) −40 cm
Answer
C
Question. Which one of the following is attached to the right ventricle?
(a) Pulmonary artery
(b) Pulmonary vein
(c) Inferior vena cava
(d) Superior vena cava
Answer
A
Question. Which of the following is the correct for dilution of acid and base?
(a) Acid or base added to water.
(b) Water is added to acid or base.
(c) Water is added drop by drop to acid or base.
(d) Water cannot be added in acid or base.
Answer
A
Question. Consider the following statements about refraction of light :
1. The incident ray, refracted ray and the normal ray lie in the same plane.
2. The angle of incidence is equal to the angle of refraction.
Choose the correct option from the codes given below:
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
A
Question. Ionic compound have high melting point due to
(a) Strong force of attraction between oppositely charged ions.
(b) Less force of attraction between oppositely charged ions.
(c) Strong force of attraction between similar charged ions.
(d) None of these
Answer
A
Question. In a combination how many products are formed?
(a) one only
(b) two only
(c) one or two only
(d) number cannot be specified
Answer
A
Question. A 4.5 cm needle is placed 12 cm away from a convex mirror of focal length 15 cm. The location of the image is-
(a) 6.7 cm
(b) 4.5 cm
(c) 9.2 cm
(d) 5 cm
Answer
A
Question. Which of the following are correctly matched?

(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
A
Question. Assertion : Active metals react with acids to liberate Hydrogen gas.
Reason : It is an example of displacement reaction.
(a) Both Assertion and Reason are true and Reason is the correct explanation of the Assertion.
(b) Both Assertion and Reason are true but Reason is not the correct explanation of the Assertion.
(c) Assertion is true but the Reason is false.
(d) Both Assertion and Reason are false.
Answer
A
Question. Which of the following substance have maximum value of pH?
(a) Lemon
(b) Rain water
(c) Sea water
(d) Apple
Answer
C
Question. Assertion : Changing of colour of copper from reddish brown to black is an example of reduction.
Reason : Hydrogen is removed.
(a) Both Assertion and Reason are True and Reason is the correct explanation of the Assertion.
(b) Both Assertion and Reason are True but Reason is not the Correct explanation of the Assertion.
(c) Assertion is True but the Reason is False.
(d) Both Assertion and Reason are False.
Answer
D
Question. In a spherical mirror, normal drawn on any point on a spherical mirror passes through :
(a) Focus
(b) Pole of the mirror
(c) Centre of curvature
(d) None of the above
Answer
C
Question. An element has a dull appearance, low density, and low melting point. It could be a _____
(a) metal
(b) non-metal
(c) both (a) and (b)
(d) none of the abvoe
Answer
C
Question. Which of the following statements is correct regarding the propagation of light of different colours of white light in air?
(a) Red light moves fastest.
(b) Blue light moves faster than green light.
(c) All the colours of the white light move with the same speed.
(d) Yellow light moves with the mean speed as that of the red and the violet light.
Answer
C
Question. Assertion : Interauricular septum separates left from right atrium.
Reason : Interventricular septum separates left from right ventricle.
(a) Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) Both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
(c) Assertion is true but Reason is false.
(d) Assertion is false but Reason is true.
Answer
B
Question. Which of the following is body’s largest blood vessel?
(a) Heart
(b) Capillaries
(c) Aorta
(d) Pulmonary vein
Answer
C
Question. Only two of the following Statements accurately describe what happens in the mouth.
1. Amylase breaks down large starch molecules into smaller maltose molecules.
2. Chewing increases the surface area of food for digestion.
3. Saliva emulsifies fats into smaller droplets.
4. Teeth breakup large insoluble molecules into smaller soluble molecules.
Which statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 3 and 4
(d) 1 and 4
Answer
A
Question. A student wants to project the image of a candle flame on a screen 80 cm in front of a mirror by keeping the candle flame at a distance of 20 cm from its pole. The magnification of the image produced is-
(a) −4
(b) −2
(c) −6
(d) −1
Answer
A
Question. What happens when a piece of zinc metal is added to copper sulphate solution?
(a) Decomposition reaction
(b) Double displacement reaction
(c) Displacement reaction
(d) Precipitation reaction
Answer
C
Question. If the refractive indices for water and diamond relative to air are 1.33 and 2.4 respectively, then the refractive index of diamond relative to water is-
(a) .55
(b) 1.80
(c) 3.19
(d) None of these
Answer
B
Question. No refraction occurs at the boundary that separates two media of equal refractive indices. Which of the following figures shows such type of refraction?


Answer
A
Question. Man is _____
(a) Ammonotelic
(b) Ureotelic
(c) Uricotelic
(d) None of the above
Answer
B
Question. A convex lens has a focal length of 10 cm. At what distance from the lens should the object be placed so that it forms a real and inverted image 20 cm away from the lens?
(a) −20 cm
(b) −40 cm
(c) −60 cm
(d) −80 cm
Answer
A
Question. Assertion : Planets do not twinkle.
Reason : Planets do not show the phenomenon of scattering.
(a) Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) Both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
(c) Assertion is true but Reason is false.
(d) Both Assertion and Reason are false.
Answer
C
Case Based Questions:
Metals are elements that exhibit a variety of physical properties such as those of malleability, ductility, conductivity of heat and electricity, lustre, etc. Due to such properties, metals find usage in purpose such as cooking utensils, machinery, modes of transportation, construction, etc., in our daily life. Metals such as gold and silver have been used in making jewellery since ancient times. Non-metals have been found to exist in all the three states– solid, liquid and gaseous. They are non-malleable, non-ductile and brittle in nature. Non-metals have very low tensile strength and are easily broken up.
Question. Metals can be given different shapes according to our needs because
(a) they are malleable and ductile
(b) they are sonorous
(c) they are generally hard
(d) they have a shining surface
Answer
A
Question. Which of the following non-metal is a good conductor of electricity?
(a) Oxygen
(b) Nitrogen
(c) Graphite
(d) Bromine
Answer
C
Question. Which of the following metal(s) will have very low melting point?
(a) Gallium
(b) Caesium
(c) Copper
(d) Both (a) and (b)
Answer
D
Question. The metal which is known as strategic metal is
(a) zirconium
(b) titanium
(c) manganese
(d) all of these
Answer
D
Case Based Questions:
They create by-products that art not only useless for the cells of the body, but could even be harmful. These waste by-products are therefore needed to be removed from the body and discarded outside by a process called excretion. Again, if the basic rules for body design in multi-cellular organisms are followed, a specialised tissue for excretion will be developed, which means that the transportation system will need to transport waste away from cells to this excretory tissue.
Question. The main excretory by-product in human beings is
(a) Creatine
(b) Urea
(c) Uric acid
(d) None of the above
Answer
B
Question. The process of removal of nitrogenous waste materials from the body is called ………. .
(a) Nutrition
(b) Respiration
(c) Excretion
(d) Transportation
Answer
C
Question. Which is the main excretory organ in human beings?
(a) Intestine
(b) Kidneys
(c) Lungs
(d) Heart
Answer
B
Question. The excretory materials are temporarily stored in:
(a) Urethra
(b) Kidneys
(c) Ureters
(d) Urinary bladder
Answer
D
Case Based Questions:
Mohan is performing an experiment with four different optical media, he traced the path of light in different media P,Q,R and S as below:


Question. Through which media, will speed of light be maximum?
(a) Q
(b) R
(c) S
(d) P
Answer
D
Question. Absolute refractive index of medium is maximum in:
(a) P
(b) Q
(c) R
(d) S
Answer
D
Question. When a light travel from medium P to S it will:
(a) reflect back to medium P
(b) pass straight without bending
(c) bend away from normal
(d) bend towards normal
Answer
D
Question. Which of the following media has maximum optical density?
(a) P
(b) R
(c) S
(d) Q
Answer
C

