Multiple Choice Questions
Question. Which of the following statements is incorrect regarding a homologous series?
(i) Compounds in a homologous series can have the same or different functional group.
(ii) Compounds in a homologous series have very less similarity in chemical properties.
(iii) Difference between the two successive compounds in a homologous series differ by a CH2 group.
(iv) Successive members in a homologous series differ in molecular mass by 14 units
(a) i and ii
(b) ii and iii
(c) iii and iv
(d) i and iv
Answer
A
Question. Name the metal that is not present in carbon family
(a) Si
(b) Ge
(c) Sb
(d) Sn
Answer
C
Question. Which of the following is unsaturated molecule?
(a) C3H8
(b) C2H2
(c) C5H12
(d) C4H10
Answer
B
Question. Which of the statement regarding homologous series is wrong?
(a) only alkanes have homologous series
(b) consecutive members of a homologous series differ by an atomic mass of 14u
(c) A given homologous series can be expressed by a general formula
(d) Homologues of a given series show gradation in physical properties.
Answer
A
Question. 3rd homologue of alkyne series is—
(a) Propyne
(b) propene
(c) butyne
(d) butane
Answer
C
Question. Which among the following is an unsaturated molecule that has the molecular formula of a cycloalkane.
(a) C3H6
(b) C8H18
(c) C5H12
(d) C3H4
Answer
A
Question. Which of the following aliphatic compounds, is saturated molecule?
(a) C6H12
(b) C2H2
(c) C5H10
(d) C4H10
Answer
D
Assertion Reason Type Questions
Question. Assertion(A): Carbon compounds can form chain, branched and ring structures.
Reason (R): Carbon exhibits the property of catenation.
Answer
A
Question. Assertion (A): Covalent compounds are generally poor conductor of electricity.
Reason (R): They consist of molecules and not ions which can transfer charge.
Answer
A
Question. Assertion (A): Graphite is a good conductor of electricity.
Reason (R): It has one free valence electron.
Answer
A
Case Study Based
1. Read the following and answer any four questions from (i) to (v)
The compounds which have the same molecular formula but differ from each other in physical or chemical properties are called isomers and the phenomenon is called isomerism. When the isomerism is due to difference in the arrangement of atoms within the molecule, without any reference to space, the phenomenon is called structural isomerism. In other words. Structural isomers are compounds that have the same molecular formula but different structural formulas, i.e., they are different in the order in which different atoms are linked. In these compounds, carbon atoms can be linked together in the form of straight chains, branched chains or even rings.
Question. Which of the following sets of compounds have same molecular formula?
(a) Butane and iso-butane
(b) Cyclohexane and hexene
(C) Propanal and propanone
(d) All of these
Answer
D
Question. In order to form branching, an organic compound must have a minimum of
(a) four carbon atoms
(b) three carbon atoms
(c) five carbon atoms
(d) any number of carbon atoms.
Answer
A
Question. Which of the following is an isomeric pair?
(a) Ethane and propane
(b) Ethane and ethene
(c) Propane and butane
(d) Butane and 2-methylpropane
Answer
D
Question. Among the following the one having longest chain is
(a) neo-pentane
(b) iso-pentane
(C) 2-methylpentane
(d) 2,2-dimethylbutane.
Answer
C
Question. The number of isomers of pentane is
(a) 2
(b) 3
(c) 4
(d) 5
Answer
B
Short Answer Type Questions
Question. A hydrocarbon molecule contains 3 carbon atoms. What would be its molecular formula in case it is (i) an alkane (ii) an alkene (iii) an alkyne?
Answer. (i) General formula of alkanes = CnH2n+2; if n = 3, formula will be C3H8
(ii) General formula of alkanes = CnH2n; So, if n = 3, formula will be C3H6
(iii) General formula of alkanes = CnH2n-2. So, if n = 3, formula will be C3H4
Question. In the electron dot structure of hydrogen molecules, each individual atom is not satisfying the octet. Justify.
Answer. For hydrogen atom as there is only a K shell,it can occupy a maximum of two electrons
Question. Define homologous series of organic compounds. List its two characteristics. Write the name and formula of the first member of the series of alkenes.
Answer. The series of organic compounds having same functional group and similar chemical properties is called homologous series. Each member differs from successive member by —CH2— group. The difference in molecular weight between two successive members is 14 u. Characteristics:
(i) It has same general formula, from which, all members can be derived.
(ii) They have similar chemical properties. C2H4, CH2 = CH2, Ethene is first member of alkene series.
Question. Allotropes of carbon has same chemical properties. Give reason.
Answer. Chemical properties of an element depends on valence electrons. Allotropes have same number of valence electrons, hence same chemical properties.
Question. An element X found in nature in solid form has 4 electrons in valence shell of its atom. Its allotrope Y has properties that allows it to be used as a dry lubricant, as also as a part of pencil lead.
(a) Identify the element.
(b) What is this allotrope Y?
(c) Write any 1 other use of this allotrope other than those mentioned here.
(d) Predict the ability of this allotrope to conduct electricity. Give reason.
(e) Name two other allotropes of this element other than Y.
Answer. (a) Carbon
(b) Graphite
(c) Used for making electrodes in dry cells.
(d) It is a good conductor of electricity.
Reason: In graphite, each carbon atom is covalently bonded with three other carbon atoms. So, only 3 valence electrons are used in bond formation and the 4th valence electron is free to move. Due to the presence of these free electrons (1 per carbon atom), graphite is a good conductor of electricity.
(e) Diamond, Buckminsterfullerene.
Question. Carbon cannot make ionic compounds. Why?
Answer. Due to small size and high effective nuclear charge, carbon cannot lose electrons to form C4+ ion and as carbon with 6 protons cannot afford four more electrons in its L shell, it cannot form C4- ions, As carbon cannot form an anion or cation, it cannot make ionic bonds.
Question. Atom of an element contains 5 electrons in the valence shell. This element exists as diatomic molecules, and is a major component of air.
(a) Identify the element.
(b) Show the bond formation between two atoms of this element.
(c) What is the nature of bond formed between the 2 atoms.
Answer. (a) Nitrogen

Question. Write the general IUPAC names of alcohol, carboxylic acid, aldehyde and ketone.
Answer.

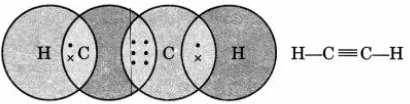
Question. Draw the electron dot structure of ethyne and also draw its structural formula.
Answer.
Question. Two elements A and B have the property C by which they can combine with more atoms of their same type. Element A is a component of the gas D that is a respiratory byproduct, while element B is the second most abundant element in the crust.
(a) Identify the elements A and B.
(b)What is the property C?
(c) Identify the gas D.
(d) Among A and B, which one shows the property C to a greater extent? Why?
Answer. (a) A is Carbon; B is Silicon.
(b) Catenation.
(c) Carbon dioxide.
(d) A (Carbon) shows greater extent of catenation than B (Silicon)Reason: Carbon atoms are smaller than that of silicon. So, carbon- carbon bonds are much stronger than silicon- silicon bonds.
Question. How many non-bonded electrons are there in?
a) Ammonia b) Methane c) Nitrogen
Answer. a) two electrons(1 pair) b) 0 c) 4(two pairs)
Question. Draw the electron dot structure of O2 and N2 molecules
Answer.

Question. a) How can you prove that butene and propane are not in a given homologous Series?
a) Name the first four homologues of alkene series?
b) How many covalent bonds are there in propene?
Answer. a) Propane has the formula C3H8 and butene is C4H8. C3H8 is of the form CnH2n+2 and belongs to alkane homologous series and C4H8 has the general formula CnH2n+2 which shows that it is an alkene. Hence those molecules are not in the same homologous series. Mention their chemical formulab) Ethene(C2H4),Propene(C3H6), Butene(C4H8),Pentene(C5H10) 7 single covalent bond and one double bond
Question. How many saturated hydrocarbons can be made using three carbon atoms? and hydrogen atoms? Name them.
Answer. Two.
Propane and cyclopropane.
Question. A hydrocarbon molecule has 4 carbon atoms. What would be its molecular formula in case it is (i) an alkane (ii) an alkene (iii) an alkyne?
Answer. (i) General formula of alkanes = CnH2n+2; if 2n+2 = 10, n = 4; so, formula will be C4H10
(ii) General formula of alkanes = CnH2n; So, if 2n = 8, n = 4; so, formula will be C4H8
(iii) General formula of alkanes = CnH2n-2. So, if 2n-2 = 6, n = 4; so, formula will be C4H6