Please refer to Hydrogen Class 11 Chemistry notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Chemistry books for Class 11. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 11 Chemistry Hydrogen Notes and Questions
✱ Position of Hydrogen in Periodic Table
➣ Lightest element known having atomic number 1.
➣ Dihydrogen
➣ It resembles both alkali metals and halogens and therefore, its position is anomalous.
➣ In modern periodic table it is located separately
➣ Resemblance with alkali metals:-
1. Electronic configuration
1H = 1s1 11Na = 1s2, 2s2, 2p6, 3s1 19K = 1s2, 2s2, 2p6, 3s23p6, 4s1
2. Electropositive character: H+, Na+, K+ etc.
3. Oxidation state: +1
4. Combination with electronegative elements: form binary compounds with electronegative elements like alkali metals.
Halides: HClNaCl, KCletc
Sulphides: H2S Na2S, K2S etc
✱ Resemblance with halogens:-
1. Electronic configuration:
Both contain one electron less than the nearest noble gas configuration
1H = 1s1 (near to 2He)
9F = 1s2, 2s2, 2p5 (near to 8Ne)
17K = 1s2, 2s2, 2p6, 3s23p5 (near to 18Ar)
2. Non-metallic character: like halogens, hydrogen is non-metallic in nature.
3. Atomicity: Diatomic molecules.
4. Formation of similar types of compounds:
i. Halides: CCl4, SiCl4, GeCl4
ii. Hydrides: CH4, SiH4, GeH4
5. Oxidation state: –1
Na+1H-1 Na+1Cl-1
✱ Difference from alkali metals:-
1) Ionization enthalpy: – the ionization enthalpy of hydrogen is very high in comparison to alkali metals.
2) Non- metallic character: alkali metals are typical metals while hydrogen is non-metal
3) Atomicity: hydrogen is diatomic while alkali metals are monoatomic.
4) Nature of compounds: the compounds of hydrogen are predominantly covalent while those of alkali metals are ionic.
For example: HCl is covalent while NaCl is ionic.
The oxides of alkali metals are basic while hydrogen oxide is neutral.
✱ Difference from halogens:-
1) Less tendency for hydride formation: Hydrogen has less tendency to take up electron to form hydride ion (H-) as compared to the halogens which from halide ions (X-) very easily.
2) Absence of unshared pairs of electrons :
3) Nature of oxides: The oxides of halogens are acidic while hydrogen oxide is neutral.
✱ Occurrence of Hydrogen:
➣ Hydrogen, the most abundant element in the universe and the third most abundant on the surface of the globe, is being visualised as the major future source of energy
✱ Isotopes of hydrogen:-
| Property | Protium | Deuterium | Tritium |
| Relative abudance | 99.99% | 0.02% | 10-15% |
| Relative atomic mass | 1.007825 | 2.014102 | 3.016 |
| Radioactive stability | Non-radioactive | Non-radioactive | Radioactive t1/2 = 12.334 yrs |
✱ Preparation:
✱ Methods for commercial production of dihydrogen
1. Electrolysis of water

➣ The hydrogen prepared by this method is of very high purity. However, this method is not commonly used because it is very expensive. This method is can be used only at those places where the electricity is cheap.
2. By the reaction of steam on coke :-

Water gas
➣ Since the mixture of CO and H2 is used for the synthesis of methanol and a number of hydrocarbons, it is also called synthesis gas or syn gas.
➣ The process of producing syn gas from coal or coke is called coal gasification

Water gas steam
➣ This reaction is called water gas shift reaction.
✱ Properties of Hydrogen:-
✱ Physical Properties:-
1) It is slightly soluble in water (about 2 %)
2) It is highly combustible and therefore should be handled carefully.
3) It lightest substance. The weight of one litre hydrogen at NTP is only 0.0899 g.
✱ Chemical properties:- Not very reactive due to high bond dissociation energy
(435.88 kJ mol-1 at 298.2 K)
(i)Combustion: – It burns with pale blue flame
2H2 (g) + O2 (g) → 2H2O(l)
(ii) Reaction with metals:- Reactive metals like Na, K, Ca, Li and form hydrides
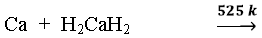
➣ Metals like Pt, Pd, Ni (elements of d block) form interstitial hydrides by absorbing large volume of hydrogen. Such hydrogen is called ‘occluded hydrogen and this property of adsorption of a gas by a metal is called occlusion.
(iii) Reaction with metal oxides:- Hydrogen reduces oxides of less active metals to corresponding metal

(iv) Reaction with non-metals:-
3H2 (g) + N2 (g) (Fe, Mo / 673k, 200 atm) -13(g) { Haber process}
ΔH = – 92.6 kj/mole
2H2 (g)+ O2 (g) 2H2OH = – 285.9 kj/i

(v) Reaction with carbon monoxide:-

(vi) Reaction with unsaturated Hydrocarbons:-

(b) Hydroformylation of olefins to aldehydes: Hydroformilation or Oxo process

(c) Hydrogenation of oils:- Vegetable oils are polyun-saturated in nature. The C =C bonds in oils can easily undergo oxidation and the oil becomes rancid i.e., unpleasant in taste. Hydrogenation reduces the number of double bonds but
completely.

✱ Uses of Hydrogen:-it itused ..
1. as a reducing agent.
2. In the manufacture of vanaspati fat, ammonia, metal hydrides, methanol, fertilizers such as urea etc.
3. In the manufacture of synthetic petrol.
4. In the atomic hydrogen torch and oxy hydrogen torches for cutting and welding. Dihydrogen is dissociated with the help of an electric arc and the hydrogen atoms produced are allowed to recombine on the surface to be welded. High temperature of about 4000 k is generated.
5. In the fuel cell for generating electrical energy.

✱ Ortho and parahydrogens :- A molecules of dihydrogen ……. abc
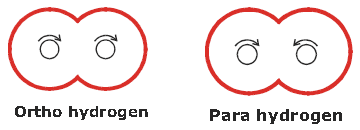
➣ They show different physical properties. For example :
(i) The thermal conductivity of para hydrogen is about 50 % greater than that of ortho hydrogen.
(ii) The melting point of para hydrogen is 0.15 k below that of hydrogen containing 75% ortho hydrogen.
➣ They show similar chemical properties.
✱ Atomic hydrogen:-
➣ Because of high H—H bond enthalpy, atomic hydrogen is produced only at high temp in an electric arc or under ultraviolet radiation.
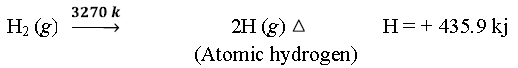
➣ Highly reactive.
➣ Half life period is 0.3 sec and therefore, it immediately gets converted into the molecular form liberating a large amount of energy which is used for cutting and welding purposes.
✱ Nascent hydrogen:- The hydrogen produced in contact with the substance to be reduced is known as ‘nascent hydrogen’. It is very reactive form of hydrogen Better reducing agent than ordinary dihydrogen.
✱ Hydrides:- Under certain conditions H2 combines with almost all the elements ,except noble gases to form compounds called hydrides.
➣ There are three types of hydrides ,they are
(i) Ionic or saline hydrides
(ii) Covalent or molecular hydrides (iii) Metallic or non-stoichiometric hydrides
(i) Ionic or saline hydrides:-
➣ These are the compounds of H2 formed with most of the s-block elements which are highly electro positive.
(ii) Covalent or molecular hydrides:-These are the compounds of hydrogen formed with most of the p-block elements
[a] Electron deficient:- The hydrides which do not have sufficient number of electrons to form normal covalent bonds is called electron deficient hydride.
For example, hydride of group 13 (BH3, AlH3, etc.).They are known as Lewis acids i.e., electron acceptors. To make up their deficiency they generally exist in polymeric forms such as B2H6, Al2H6, etc.
[b] Electron precise:- The hydrides which have sufficient number of electrons required for forming covalent bonds is called electron precise hydride.
For example, hydrides of group 14 (CH4, SiH4, GeH4, SnH4, PbH4 etc.) they have tetrahedral geometry.
[c] Electron rich hydrides:-The hydrides which have excess electrons as required to form normal covalent bonds is called electron rich hydride.
For example, hydrides of group 15 to 17 (NH3, PH3, H2O, H2S, H2Se, H2Te, HF etc.)
(iii) Metallic or non-stoichiometric hydrides:-
➣ These are formed by many d-block and f-block elements
➣ These hydrides conducts heat and electricity though not efficient.
✱ Water: – Water! It is the major part of all living organisms. water is also known as the river of life.
➣ Human body has about 65%and some plants have as much as 95%water.
✱ Structure Of Water:-
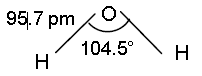
➣ In a gas phase water is bent molecule with a bond angle of 104.5 and O-H bond length of 95.7pm It is highly polar molecule.

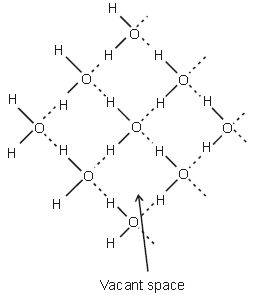
✱ Structure of ice:-Ice has a highly ordered 3D hydrogen bonded structure. Each oxygen atom is surrounded tetrahedrally by four other four other oxygen atoms at a distance of 276 pm
✱ Chemical Properties of water:-
[i] Amphoteric Nature:- It has the ability to act as an acid as well as base .it acts as an acid with NH3 and as a base with H2S
H2O(l) +NH3(aq) → OH-(aq) +NH4 + (aq)
H2O(l) +H2S(aq) → H3O+(aq) +HS– (aq)
[ii] Redox Reactions Involving Water:-
➣ Water can be easily reduced to H2 by highly electropositive metals
2H2O(l) +2Na(s) → 2NaOH(aq) +H2(g)
[iii] Hydrolysis Reaction: Due to high dielectric constant, it has a very strong hydrating tendency .it dissolves many ionic compounds
P4O10(s) +6H20(l) → 4H3PO4(aq)
SiCl4(l) +2H2O(l) → SiO2(s) + 4HCl(aq)
[Iv] HYDRATES FORMATION:- From the aqueous solutions many salts can be crystallized as hydrated salts. It of different types.
(1) Coordinated water e.g., [Cr(H2O)6]3+ 3Cl-
(2) Interstitial water e.g.,BaCl2.2H2O
(3) hydrogen-bonded water e.g. [Cu(H2O)4]2+ 4SO2- .H2Oin CuSO4.5H2O.
✱ Hard & Soft Water:-
➣ The water which contains dissolved salts of bicarbonates, sulphates and chlorides of calcium and magnesium is called hard water. Hard water does not produce lather with soap solution.
➣ Soft water is free from bicarbonates, sulphates and chlorides of calcium and magnesium. It produces lather with soap solution easily. e.g., distilled water, rain water..
✱ Types of hardness:- The hardness of water is of two types
(i) Temporary hardness
➣ Due to presence of soluble bicarbonates of calcium and magnesium.
➣ Can be removed by simple boiling.
(ii) Permanent hardness
➣ Due to presence of chlorides and sulphates of calcium and magnesium.
➣ Requires treatment of water to remove this type hardness.
➣ Do you know?
Temporary hardness is also called carbonate hardness & Permanent hardness is also called non-carbonate hardness
✱ Softening of water:- The process of removal of Ca2+ and Mg2+ ions from water is called softening of water.
✱ Removal of temporary hardness:-
(i) By boiling :

(ii) Clark’s method or calcium hydroxide method
Ca(HCO3)2 + Ca(OH)2 →2CaCO3 + 2H2O
(Soluble) (Insoluble)
Mg(HCO3)2 + 2Ca(OH)2 → 2CaCO3 + Mg(OH)2 + 2H2
(Soluble) (Insoluble)(Insoluble)
✱ Removal of permanent hardness:-
(i) By washing soda (Na2CO3.10H2O) treatment:
CaCl2 + Na2CO3 → 2CaCO3 + 2NaCl
(Insoluble)
MgSO4 + Na2CO3 ――→ MgCO3 + Na2SO4
(Insoluble)
(ii) By using inorganic cation exchanger (permutit method or Zeolite method):
Na2Al2Si2O8 + CaCl2 → Ca(Al2Si2O8)2 + 2NaCl
Zeolite Settles at bottom
The zeolite can be regenerated by treatment with sodium chloride solution.
Ca(Al2Si2O8)2 + 2NaCl → Na2Al2Si2O8 + CaCl2
(iii) By organic ion exchanger or synthetic resins (ion exchange resins):-
➣ Synthetic resins are the insoluble polymeric solids having giant hydrocarbon network containing reactive acidic or basic groups. These are superior to Zeolite because they can remove all types of cations as well as anions present in
water. This resulting water is known as demineralised or deionised water.
➣ These are two types:
(a) Cation exchanger resins: they have acidic groups such as COOH or SO3H.
they may be represented as resin —H+
Mg2+ + 2H – resin → mg (resin)2 + 2H+
In hard water caption exchanger
Ca2+ + 2H – resin → Ca (resin)2 + 2H+
hard water caption exchanger
(b) Anion exchanger resins: they have basic groups such as –OH– or – NH2. they may be represented as resin— OH– or resin — NH3 + OH–
SO2-4 + 2HO – resin → SO4 – (resin)2 + 2OH+
hard water Anion exchanger
CI– + HO – resin → CI – (resin) + OH–
hard water Anion exchanger
Regeneration of resiners:
CI- resin + NaOH → HO – resin + NaCI
Exchausted resin Re generated resin
✱ Hydrogen peroxide H2O2 ]:- discovered by French chemist J.L. The nard
✱ Methods of preparation
1) From sodium peroxide (Merck’s process):-
Na2O2 + H2SO4 → Na2SO4 + H2O2
(20% ice cooled solution) (30% solution)
2. From Barium peroxide:-
➣ Hydrogen peroxide was first prepared by J. L. Thenard in 1818 by acidifying barium peroxide and removal of excess water by evaporation under reduced pressure.
BaO2.8H2O + H2SO4 BaSO4 + 8H2O + H2O2
➣ Barium sulphate is filtered off leaving behind H2O2 .
Store of Hydrogen peroxide:-
a) Itmust be kept in wax lined coloured bottles because the rough glass surface causes its decomposition.
b) A small amount of phosphoric acid , glycerol or acetanilide is generally added which retard the decomposition of H H2O2 . These are also called negative catalysts.
✱ Physical properties of Hydrogen peroxide:-
1. In the pure state H2O2 is an almost colourless(very pale blue) liquid.
2. H2O2 is miscible with water in allproportions and forms a hydrate H2O2 .H2O (mp 221K).
3. A 30% solution of H2O2 is marketedas ‘100 volume’ hydrogen peroxide. It means thatone millilitre of 30% H2O2 solution will give 100 Vof oxygen at STP. Commercially, it is marketedas 10 V, which means it contains 3% H2O2 .
Chemical properties of Hydrogen peroxide:-

✱ Uses of hydrogen peroxide
1) For bleaching silk, wool, hair and leather
2) As rocket fuel
✱ Structure of hydrogen peroxide

Hydrogen economy (Hydrogen as fuel)
☞ The electricity cannot be stored to run automobiles. It is not possible to store and transport nuclear energy. Hydrogen is an alternative source of energy and hence called as ‘hydrogen economy’. Hydrogen has some advantages as fuel
☞ Available in abundance in combined form as water.
☞ On combustion produces H2O. Hence pollution free.
☞ H2 – O2 fuel cell give more power.
☞ Excellent reducing agent. Therefore can be used as substitute of carbon in reduction for processes in industry.
✱ Obstacles in hydrogen economy
☞ Transportation:
☞ Hydrogen gas is explosive and hence it is difficult to store and transport.
☞ Formation of hydrogen from H2O:
☞ The cheaper production of the hydrogen is basic requirement of hydrogen economy which is not possible now.
☞ The main aim and advantage of hydrogen economy is to transmit energy in four of hydrogen.
Important Questions Hydrogen
Question. What is meant by 10 volume hydrogen peroxide?
Ans. It means that 1 ml of H2O2 will give 10 ml of oxygen at N.T.P.
Question. Why is dihydrogen gas not preferred in balloons?
Ans. Dihydrogen gas is combustible in nature. Therefore, it may react with oxygen highly violently. Thus, it is not used in balloons.
Question. Name the constituents of water gas.
Ans. Carbon monoxide and hydrogen.
Question. Name one compound each in which hydrogen exists in (i) positive oxidation state, and (ii) Negative oxidation state.
Ans. (i) HCl (ii) NaH
Question. What type of elements form interstitial hydrides?
Ans. Elements of d-and f-block.
Question. How many hydrogen bonded water molecule(s) are present in CuSO4.5H2O?
Ans. In CuSO4.5H2O, there is one hydrogen bonded water molecule which is outside the coordination sphere. The other four molecules of water are coordinated.
Question. What happens when heavy water is added to calcium carbonate?
Ans. Deutero acetylene is formed.
CaC2 + 2D2O Ca(OD)2 + C2D2
Question. Concentrated sulphuric acid cannot be used for drying H2. Why?
Ans. Conc. H2SO4 on absorbing water from moist H2 produces so much heat that H2 catches fire
Question. Complete the following reactions?
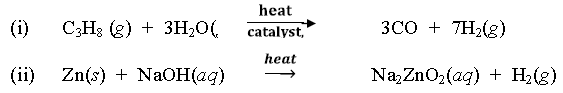
Question. How heavy water is is obtained from ordinary water?
Ans. Heavy water is obtained from ordinary water by repeated electrolysis in the presence of 3% NaOH.
Question. Can we use concentrated sulphuric acid and pure zinc in the preparation of dihydrogen?
Ans. (a) Conc. H2SO4 cannot be used because it acts as oxidizing agent also and gets reduced to SO2.
Zn + dil H2SO4 ZnSO4 + 2H2O + SO2
(b) Pure Zn is not used because it is non-porous and reaction will be slow. The impurities in Zn help in constitute of electrochemical couple and speed up reaction.
Question. Write the chemical reactions to show the amphoteric nature of water.
Ans. Water is amphoteric in nature and it behaves both as an acid as well as a base. With acids stronger than itself (eg., H2S) it behaves as a base and with bases stronger than itself (eg. NH3) it acts as an acid.
(i) As a base: H2O(l) +H2S(aq) → H3O+(aq) +HS- (aq)
(ii) As an acid: H2O(l) +NH3(aq) → OH-(aq) +NH4 + (aq)
Question. Why is hydrogen peroxide stored in wax-lined plastic coloured bottles?
Ans. The decomposition of H2SO4 occurs readily in the presence of rough surface (acting as catalyst). It is also decomposed by exposure of light. Therefore, waxlined smooth surface and coloured bottles retard the decomposition of H2SO4 .
Question. H2SO4 acts as an oxidizing agent as well as reducing agent. Why?
Ans. In H2SO4 , oxygen has -1 oxidation state which lies between maximum (0 or +2 in OF2) and minimum -2. Therefore, oxygen can be oxidized to O2 (zero oxidation state) acting as reducing agent or can be reduced to H2O or OH- (-2 oxidation state) acting as an oxidizing agent.
-1 0
O2→O2+ 2e-
(Reducing agent)
-1 -2
O2 + 2e- → 2O
(Oxidizing agent)
Question. What causes the temporary and permanent hardness of water?
Ans. Temporary hardness is due to presence of soluble bicarbonates of calcium and magnesium. On the other hand, permanent hardness is due to presence of chlorides and sulphates of calcium and magnesium.
Question. Hard water is unsuitable for laundry, washing and dyeing. Explain.
Ans. since we know that the soap are the sodium salts of higher fatty acids like stearic acid (C17H35COOH), oleic acid (C1
Question. Hard water is unsuitable for laundry, washing and dyeing. Explain.
Ans. since we know that the soap are the sodium salts of higher fatty acids like stearic acid (C17H35COOH), oleic acid (C17H33COOH) or palmitic acid (C17H31COOH). When soap is added to hard water, the anions of soap combine with Ca+2 and Mg+2 ions to form calcium and magnesium salt which are insoluble in water.
M+2 + 2C17H35COONa → (C17H35COO)2M + 2Na
From hard sodium stearate Metal stearate
Water (Soap) (precipitate)
Therefore, no lather is produce until all the calcium and magnesium ions are precipitated. This also results into wastage of lot of soap. So hard water isunsuitable for laundry, washing and dyeing.
Question. What do you understand by (i) electron-deficient, (ii) electron – precise, and (iii) electron – rich compounds of hydrogen? Provide justification with suitable examples.
Ans. [a]Electron deficient :- The hydrides which do not have sufficient number of electrons to form normal covalent bonds is called electron deficient hydride.
For example, hydride of group 13 (BH3, AlH3, etc.).
[b] Electron precise :- The hydrides which have sufficient number of electrons required for forming covalent bonds is called electron precise hydride. For example, hydrides of group 14 (CH4, SiH4, GeH4, SnH4, PbH4 etc.) they have tetrahedral geometry.
[c] Electron rich hydrides :- The hydrides which have excess electrons as required to form normal covalent bonds is called electron rich hydride. For example, hydrides of group 15 to 17 (NH3, PH3, H2O, H2S, H2Se, H2Te, HF etc.)
Question. Compare the structures of H2O and H2O2.
Ans. In water, O atom is sp3hybridised and there are two O—H bonds and two sp3 hybrid orbitals occupy lone pairs of electrons. Due to stronger lone pair-lone pair repulsions than bond pair-bond pair repulsions, the H-O-H bond
decreases from 109.5 to 104.50. Therefore, water molecule is a bent or angular molecule. H2O2 has non-planar structure. In this structure, two O—O oxygen atoms are bonded to each other by a single covalent bond and each O atom is further bonded to a hydrogen atom by a single covalent bond. The two O—H bonds are in different planes in 111.50 in the gas phase.




