Check the below Atoms and Molecules Class 9 MCQ for Science Chapter 3 with Answers available with PDF free download. MCQ Questions for Class 9 Science with Answers were prepared based on the latest syllabus and examination pattern issued by CBSE, NCERT and KVS. Our teachers have provided below Atoms and Molecules Class 9 Science MCQs Questions with answers which will help students to revise and get more marks in exams
Atoms and Molecules Class 9 Science MCQ Questions with Answers
Refer below for Atoms and Molecules Class 9 MCQ with solutions. Solve MCQ questions and compare them with the answers provided below
Question. The atomicities of ozone, sulphur, phosphorus and argon are respectively :
(a) 8, 3, 4 and 1
(b) 1, 3, 4 and 8
(c) 4, 1, 8 and 3
(d) 3, 8, 4 and 1
Answer
D
Question: The total number of electrons present in 16 g of methane gas is
(a) 96.352 × 1023
(b) 48.176 × 1023
(c) 60.22 × 1023
(d) 30.110 × 1023
Answer
C
Question: Which of the following weighs most?
(a) 2 g atoms of nitrogen
(b) 25 g iron
(c) 2×× 1023 atoms of carbon
(d) 1 mole of SO2
Answer
D
Question: The formula of chloride of a metal M is MCl3,then the formula of the phosphate of metal Mwill be
(a) MPO4
(b) M2PO4
(c) M3PO4
(d) M2(PO4)3
Answer
A
Question: Which of the following has highest number of molecules?
(a) 8 g of CH4
(b) 4.4 g of CO2
(c) 34.2 g of C12H22O11
(d) 2 g of H2
Answer
D
Question: All samples of carbon dioxide contain carbon and oxygen in the mass ratio 3 : 8. This is in agreement with the law of
(a) conservation of mass
(b) constant proportions
(c) multiple proportions
(d) gaseous volumes.
Answer
B
Question: The given figure shows the set-up t o study the reaction between gas X and copper (II) oxide:
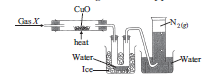
Which of the following statements is/are correct?
(Given : Atomic mass of N = 14 u, H = 1 u, O = 16 u,C = 12 u)
I. Gas X is a compound of two elements,nitrogen and hydrogen.
II. The number of atoms present in 0.5 mole of N2 is 6.023 ×1023.
III. 1 mole of H2O contains 1 mole of oxygen molecules and 2 moles of hydrogen atoms.
(a) I and II only
(b) I and III only
(c) II only(d) III only
Answer
A
Question: It was found that 0.10 mole of MSO4combines with 9.0 g of water to form the hydrate salt MSO4.nH2O. What is the value of n?
(a) 2
(b) 3
(c) 4
(d) 5
Answer
D
Question: Two gaseous samples were analyse(d) One contained 1.2 g of carbon and 3.2 g of oxygen. The other contained 27.3% carbon and 72.7% oxygen.
The experimental data are in accordance with
(a) law of conservation of mass
(b) law of definite proportions
(c) law of reciprocal proportions
(d) law of multiple proportions.
Answer
B
Question: 3.42 g of sucrose are dissolved in 18 g of water in a beaker. The number of oxygen atoms in the solution are
(a) 6.68 × 1023
(b) 6.09 × 1022
(c) 6.022 × 1023
(d) 6.022 × 1021
Answer
A
Question: Which of the following correctly represents 360 g of water?
(i) 2 moles of H2O
(ii) 20 moles of water
(iii) 6.022 × 1023 molecules of water
(iv) 1.2044 × 1025 molecules of water
(a) (i)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
D
Question: Arrange the following in the increasing order of mass in grams :
(i) One atom of silver
(ii) Two grams atom of nitrogen
(iii) One mole of calcium
(iv) Two grams of sodium
[At. masses : Ag = 108 u, N = 14 u, Ca = 40 u, Na = 23 u]
(a) (i) < (ii) < (iii) < (iv)
(b) (iv) < (iii) < (ii) < (i)
(c) (i) < (iv) < (ii) < (iii)
(d) (iii) < (ii) < (i) < (iv)
Answer
C
Question: The molecular mass of X is 106. X among the following is
(a) CaCO3
(b) SO3
(c) Na2CO3
(d) NaCl
Answer
C
Question. In water, the proportion of oxygen and hydrogen by mass is :
(a) 1 : 4
(b) 1 : 8
(c) 4 : 1
(d) 8 : 1
Answer
D
Question. The symbols of the elements cobalt, aluminium, helium and sodium respectively written by a student are as follows. Which symbol is the correct one ?
(a) CO
(b) AL
(c) He
(d) So
Answer
C
Question. The symbol of a metal element which is used in making thermometers is :
(a) Ag
(b) Hg
(c) Mg
(d) Sg
Answer
B
Question. The Latin language name of an element is natrium. The English name of this element is :
(a) sodium
(b) potassium
(c) magnesium
(d) sulphur
Answer
A
Question. The atomic theory of matter was proposed by :
(a) John Kennedy
(b) Lavoisier
(c) Proust
(d) John Dalton
Answer
D
Question. One of the following elements has an atomicity of ‘one’. This element is :
(a) helium
(b) hydrogen
(c) sulphur
(d) ozone
Answer
A
Question. The English name of an element is potassium, its Latin name will be :
(a) plumbum
(b) cuprum
(c) kalium
(d) natrium
Answer
C
Question. The law of conservation of mass was given by :
(a) Dalton
(b) Proust
(c) Lavoisier
(d) Berzelius
Answer
C
Question. If 1.4 g of calcium oxide is formed by the complete decomposition of calcium carbonate, then the amount of calcium carbonate taken and the amount of carbon dioxide formed will be respectively :
(a) 2.2 g and 1.1 g
(b) 1.1 g and 2.5 g
(c) 2.5 g and 1.1 g
(d) 5.0 g and 1.1 g
Answer
C
Question. The law of constant proportions was given by :
(a) Proust
(b) Lavoisier
(c) Dalton
(d) Berzelius
Answer
A
Question. The element having atomicity ‘four’ is most likely to be :
(a) argon
(b) fluorine
(c) phosphorus
(d) francium
Answer
C
Question. The scientist who proposed the first letter (or first letter and another letter) of the Latin or English name of an element as its symbol, was :
(a) Dalton
(b) Proust
(c) Lavoisier
(d) Berzelius
Answer
D
Question. Which of the following elements has the same molecular mass as its atomic mass ?
Question(a) nitrogen
(b) neon
(c) oxygen
(d) chlorine
Answer
B
Question. Out of ozone, phosphorus, sulphur and krypton, the elements having the lowest and highest atomicities are respectively :
(a) sulphur and krypton
(b) krypton and ozone
(c) phosphorus and sulphur
(d) krypton and sulphur
Answer
D
Question. One nm is equal to :
(a) 10–9 mm
(b) 10–7 cm
(c) 10–9 cm
(d) 10–6 m
Answer
B
Question. In hydrogen peroxide (H2O2), the proportion of hydrogen and oxygen by mass is :
(a) 1 : 8
(b) 1 : 16
(c) 8 : 1
(d) 16 : 1
Answer
B
Question. The atoms of which of the following pair of elements are most likely to exist in free state ?
(a) hydrogen and helium
(b) argon and carbon
(c) neon and nitrogen
(d) helium and neon
Answer
D
Question. ‘If 100 grams of pure water taken from different sources is decomposed by passing electricity, 11 grams of hydrogen and 89 grams of oxygen are always obtained’. Which chemical law is illustrated by this statement ?
Answer
Law of constant proportions
Question. State whether the following statement is true or false :
The symbol of element cobalt is CO.
Answer
False
Question. The radius of an oxygen atom is 0.073 nm. What does the symbol ‘nm’ represent ?
Answer
nanometre (10–9 m)
Question. ‘If 100 grams of calcium carbonate (whether in the form of marble or chalk) are decomposed completely, then 56 grams of calcium oxide and 44 grams of carbon dioxide are obtained’. Which law of chemical combination is illustrated by this statement ?
Answer
Law of conservation of mass
Fill in the following blanks with suitable words :
Question In a chemical reaction, the sum of the masses of the reactants and the products remains unchanged. This is called………….. .
Answer
conservation of mass
Very Short Answer Type Questions
Question: How many H atoms are in 0.80 moles of hexane, C6H14?
Answer: Number of hexane molecules
= Number of moles × 6.022 × 1023
= 0.8 × 6.022 × 1023 = 4.81 × 1023 One
hexane molecule contains 14 H atoms Hence, total number of H atoms in 0.8 moles = 4.8 × 1023 × 14
= 67.2 × 1023 = 6.72 × 1024 atoms
Question: Define law of conservation of mass.
Answer: Law of conservation of mass states that mass can neither
be created nor be destroyed in a chemical reaction.
Question: Formula of the carbonate of a metal M is M2CO3. Write the formula of its chloride.
Answer: In MCl valency of M is 1.
Question: Define the atomicity of a molecule of an element?
Answer: The number of atoms present in one molecule of an element is called its atomicity.
Question: Calculate formula mass of sodium carbonate.
Answer: The formula of sodium carbonate is Na2CO3.
∴ Formula mass of sodium carbonate
= 23 × 2 + 12 + 3 × 16 = 46 + 12 + 48 = 106
Question: Calculate the mass of 1.12 moles of sulphur trioxide molecules.
Answer: Relative molecular mass of SO3 = 1(32) + 3(16) = 80
Molar mass of SO3 = 80 g/mol
Mass of 1.12 moles of SO3
= Number of moles × Molar mass
= 1.12 mol × 80 g/mol = 89.6 g
Question: Give one example of (i) a polyatomic cation, (ii) a polyatomic anion.
Answer: (i) NH+4, (ii) SO2-4
Question: Write the formulae for the following :
a. Calcium phosphate
b. Magnesium hydroxide
Answer:
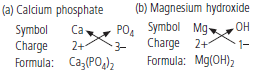
Case Based MCQs
The knowledge of valencies of various radicals helps us to write the formulae of chemical compounds. The total positive charge on positive ions (cations) is equal to the total negative charge on negative ions (anions) in a molecule.
Therefore, in writing the formula of a compound,the positive and negative ions are adjusted in such a way that the total number of positive charges of positive ions (cations) becomes equal to the total number of negative charges of negative ions (anions).
There is another simple method for writing the formulae of ionic compounds. In this method, the valencies (or positive or negative charges) of the ions can be ‘crossed over’ to give subscripts. The purpose of crossing over of charges is to find the number of ions required to equalise the number of positive and negative charges.
Question: The formula of the sulphate of an element X is X2(SO4)3. The formula of nitride of element X will be
(a) X2N
(b) XN2
(c) XN
(d) X2N3
Answer
C
Question: Element X has two valencies 5 and 3 and Y has valency 2. The elements X and Y are most likely to be respectively
(a) copper and sulphur
(b) sulphur and iron
(c) phosphorus and fluorine
(d) nitrogen and iron.
Answer
D
Question: The formula of a compound is X3Y. Thevalencies of elements X and Y will be respectively
(a) 1 and 3
(b) 3 and 1
(c) 2 and 3
(d) 3 and 2
Answer
A
A mole of an atom is a collection of atoms whose total mass is the number of grams equal to the atomic mass. Since equal number of moles of different elements contain an equal number of atoms it becomes convenient to express the amounts of the elements in terms of moles. A mole represents a definite number of particles viz, atoms, molecules, ions or electrons. This
definite number is called Avogadro number or Avogadro constant which is equal to 6.022 × 1023. Hence a mole represents 6.022 × 1023particles of the substance. One mole of substance represents one gram-formula of the substance.
One mole of a gas at standard temperature and pressure occupies 22.4 litres.
Question: What is the mass in grams of a single atom of chlorine? (Atomic mass of chlorine = 35.5)
(a) 6.54 × 1023 g
(b) 5.9 × 10–23 g
(c) 0.0025 g
(d) 35.5 g
Answer
B
Question: What is the mass in grams of 2.42 mol of zinc? (Atomic mass of Zn = 65.41)
(a) 200 g
(b) 25 g
(c) 85 g
(d) 158 g
Answer
D
Question: How many grams of sodium must be taken to get 1 mole of the element ?
(a) 23 g
(b) 35.5 g
(c) 63.5 g
(d) 46 g
Answer
A
Question: How many number of moles are there in 5.75 g of sodium ?
(Atomic mass of sodium = 23)
(a) 0.25
(b) 0.5
(c) 1
(d) 2.5
Answer
A


