Students can read the important questions given below for Structure Of The Atom Class 9 Science. All Structure Of The Atom Class 9 Notes and questions with solutions have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS. You should read all notes provided by us and Class 9 Science Important Questions provided for all chapters to get better marks in examinations. Science Question Bank Class 9 is available on our website for free download in PDF.
Important Questions of Structure Of The Atom Class 9
Question. Which of the following correctly represent the electronic distribution in the Mg atom?
(a) 3, 8, 1
(b) 2, 8, 2
(c) 1, 8, 3
(d) 8, 2, 2
Answer
B
Question. Which of the following are true for an element?
(i) Atomic number = number of protons + number of electrons
(ii) Mass number = number of protons + number of neutrons
(iii) Atomic mass = number of protons = number of neutrons
(iv) Atomic number = number of protons = number of electrons
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
D
Question. The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
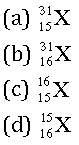
Answer
A
Question. In a sample of ethyl ethanoate (CH3COOC2H5) the two oxygen atoms have the same number of electrons but different number of neutrons. Which of the following is the correct reason for it?
(a) One of the oxygen atoms has gained electrons
(b) One of the oxygen atoms has gained two neutrons
(c) The two oxygen atoms are isotopes
(d) The two oxygen atoms are isobars.
Answer
C
Question. Rutherford’s ‘alpha (α) particles scattering experiment’ resulted in to discovery of
(a) Electron
(b) Proton
(c) Nucleus in the atom
(d) Atomic mass
Answer
C
Question. In the Thomson’s model of atom, which of the following statements are correct?
(i) the mass of the atom is assumed to be uniformly distributed over the atom
(ii) the positive charge is assumed to be uniformly distributed over the atom
(iii) the electrons are uniformly distributed in the positively charged sphere
(iv) the electrons attract each other to stabilize the atom
(a) (i), (ii) and (iii)
(b) (i) and (iii)
(c) (i) and (iv)
(d) (i), (iii) and (iv)
Answer
A
Question. Which of the following statements about Rutherford’s model of atom are correct?
(i) considered the nucleus as positively charged
(ii) established that the α–particles are four times as heavy as a hydrogen atom
(iii) can be compared to solar system
(iv) was in agreement with Thomson’s model
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) only (i)
Answer
A
Question. Rutherford’s α–particle scattering experiment showed that
(i) electrons have negative charge
(ii) the mass and positive charge of the atom is concentrated in the nucleus
(iii) neutron exists in the nucleus
(iv) most of the space in atom is empty
Which of the above statements are correct?
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer
B
Question. Dalton’s atomic theory successfully explained
(i) Law of conservation of mass
(ii) Law of constant composition
(iii) Law of radioactivity
(iv) Law of multiple proportion
(a) (i), (ii) and (iii)
(b) (i), (iii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (ii) and (iv)
Answer
D
Question. Identify the Mg2+ ion from the Fig.4.1 where, n and p represent the number of neutrons and protons respectively

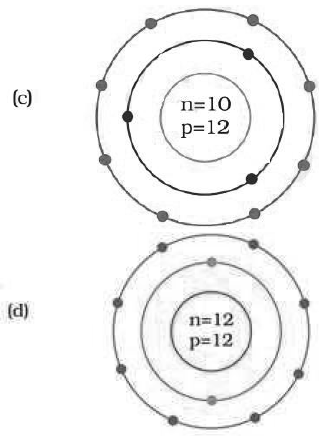
Answer
D
Question. Elements with valency 1 are
(a) always metals
(b) always metalloids
(c) either metals or non-metals
(d) always non-metals
Answer
C
Question. Which of the following statement is always correct?
(a) An atom has equal number of electrons and protons.
(b) An atom has equal number of electrons and neutrons.
(c) An atom has equal number of protons and neutrons.
(d) An atom has equal number of electrons, protons and neutrons.
Answer
A
Question. The first model of an atom was given by
(a) N. Bohr
(b) E. Goldstein
(c) Rutherford
(d) J.J. Thomson
Answer
D
Question. The ion of an element has 3 positive charges. Mass number of the atom is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
(a) 13
(b) 10
(c) 14
(d) 16
Answer
B
Question. An atom with 3 protons and 4 neutrons will have a valency of
(a) 3
(b) 7
(c) 1
(d) 4
Answer
C
Question. Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?

(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (i) and (iv)
Answer
C
Question. Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(iii) Bohr’s atomic model
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (i)
(c) (ii), (i) and (iii)
(d) (iii), (ii) and (i)
Answer
C
Question. The electron distribution in an aluminium atom is
(a) 2, 8, 3
(b) 2, 8, 2
(c) 8, 2, 3
(d) 2, 3, 8
Answer
A
Fill in the blanks in the following statements
Question. The electronic configuration of silicon is __________ and that of Sulphur is __________.
Answer
Silicon—2, 8, 4, Sulphur— 2, 8, 6
Question. Isotopes have same __________ but different __________.
Answer
atomic number, mass number
Question. Neon and chlorine have atomic numbers 10 and 17 respectively. Their valencies will be __________ and __________ respectively.
Answer
0 and 1.
Question. Rutherford’s α-particle scattering experiment led to the discovery of the __________.
Answer
atomic nucleus
Question. Why did Rutherford select a gold foil in his α–ray scattering experiment?
Ans. Gold is highly malleable. Hence, it can be made into very thin sheet. Rutherford wanted a metal sheet which could be as thin as possible. So, he selected gold foil for his alpha-ray scattering experiment.
Question. Write any two observations which support the fact that atoms are divisible.
Ans. Discovery of electrons and protons
Question. Is it possible for the atom of an element to have one electron, one proton and no neutron. If so, name the element.
Ans. Yes, it is true for hydrogen atom which is represented as 11H
Question. In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed?
Answer. This element needs to gain two electrons in order to acquire noble gas configuration. So, the charge on ion would be – 2.
Question. Will 35Cl and 37Cl have different valencies? Justify your answer.
Ans. They are isotopes. Isotopes have same number of electrons in them. Hence, their valencies do not differ.
Question. Find out the valency of the atoms represented by the Fig. 4.3 (a) and (b).

Answer. Atom ‘a’ has zero valency; while atom ‘b’ has a valency of 1.
Question. Write down the electron distribution of chlorine atom. How many electrons are there in the L shell? (Atomic number of chlorine is 17).
Ans. 2, 8, 7. The L shell has eight electrons
Question. Calculate the number of neutrons present in the nucleus of an element X which is represented as 3115X.
Answer. Mass number = No. of protons + No. of neutrons = 31
∴ Number of neutrons = 31 – number of protons
= 31 – 15 = 16
Question. What information do you get from the Fig. 4.4 about the atomic number, mass number and valency of atoms X, Y and Z? Give your answer in a tabular form.
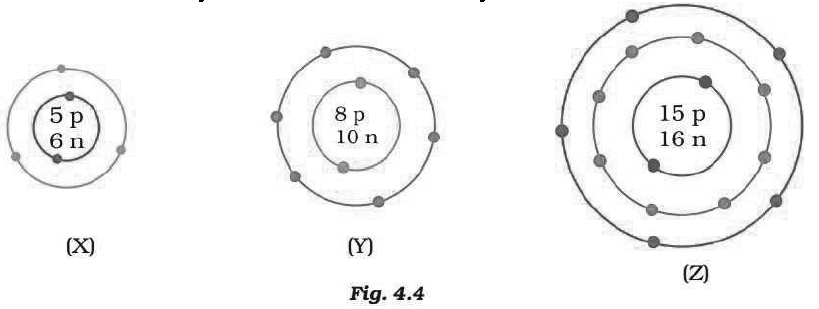
Answer.

Question. The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
Answer. Elements with same mass number and different atomic numbers are called isobars.
Question. One electron is present in the outer most shell of the atom of an element X. What would be the nature and value of charge on the ion formed if this electron is removed from the outer most shell?
Ans. Single and positive charge (+1).
Question. Match the names of the Scientists given in column A with their contributions towards the understanding of the atomic structure as given in column B

Answer. (a) (iii)
(b) (iv)
(c) (i)
(d) (ii)
(e) (vi)
(f) (vii)
(g) (v)
Question. An element X has a mass number 4 and atomic number 2. Write the valency of this element?
Answer. Valency is zero as K shell is completely filled.
Question. Complete the Table 4.1 on the basis of information available in the symbols given below
(a) 3517CI
(b) 126C
(c) 8135Br

Answer.

Question. Helium atom has 2 electrons in its valence shell but its valency is not 2, Explain.
Answer. Helium atom has 2 electrons in its outermost shell and its duplet is complete. Hence the valency is zero.
Question. What were the drawbacks of Rutherford’s model of an atom?
Answer. The orbital revolution of the electron is not expected to be stable. Any particle in a circular orbit would undergo a acceleration and the charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know.
Question. Why do Helium, Neon and Argon have a zero valency?
Answer. Helium has two electrons in its only energy shell, while Argon and Neon have 8 electrons in their valence shells. As these have maximum number of electrons in their valence shells, they do not have any tendency to combine with other elements. Hence, they have a valency equal to zero.
Question. In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons. Do you agree with the statement? Justify your answer.
Answer. Student is giving wrong statement. Number of protons can never be more than that of neutrons. It can be equal to or less than the number of neutrons. Number of electrons and protons is always equal in an atom.
Question. The ratio of the radii of hydrogen atom and its nucleus is ~ 105. Assuming the atom and the nucleus to be spherical, (i) what will be the ratio of their sizes? (ii) If atom is represented by planet earth ‘Re’ = 6.4 ×106 m, estimate the size of the nucleus.
Answer. (i) Volume of the sphere = 4/3 πr3
Let R be the radius of the atom and r be that of the nucleus.
⇒ R=105 r

(ii) If the atom is represented by the planet earth (Re = 6.4 x 106 m) then the radius of the
nucleus would be rn = Re/105
rn = 6.4 x 106m/105
= 6.4 ×10m
= 64 m.
Question. In what way is the Rutherford’s atomic model different from that of Thomson’s atomic model?
Answer. Rutherford proposed a model in which electrons revolve around the nucleus in welldefined orbits. There is a positively charged centre in an atom called the nucleus. He also proposed that the size of the nucleus is very small as compared to the size of the atom and nearly all the mass of an atom is centred in the nucleus. Whereas, Thomson proposed the model of an atom to be similar to a christmas pudding. The electrons are studded like currants in a positively charged sphere like christmas pudding and the mass of the atom was supposed to be uniformly distributed.
Question. What are the postulates of Bohr’s model of an atom?
Answer. The postulates put forth by Neils Bohr’s about the model of an atom:
(i) Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
(ii) While revolving in discrete orbits the electrons do not radiate energy. These orbits are called energy levels. Energy levels in an atom are shown by circles. These orbits are represented by the letters K, L, M, N, … or the numbers, n = 1, 2, 3, 4, ….
Question. Show diagramatically the electron distributions in a sodium atom and a sodium ion and also give their atomic number.
Answer.

Since the atomic number of sodium atom is 11, it has 11 electrons. A positively charged sodium ion (Na+) is formed by the removal of one electron from a sodium atom. So, a sodium ion has 11–1 = 10 electrons in it. Thus, electronic distribution of sodium ion will be 2, 8. The atomic number of an element is equal to the number of protons in its atom. Since, sodium atom and sodium ion contain the same number of protons, therefore, the atomic number of both is 11.
Question. Enlist the conclusions drawn by Rutherford from his α-ray scattering experiment
Answer. Rutherford concluded from the α-particle scattering experiment that–
(i) Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
(ii) Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
(iii) A very small fraction of α-particles were deflected by 180o, indicating that all the positive charges and mass of the gold atom were concentrated in a very small volume within the atom. From the data he also calculated that the radius of the nucleus is about 105 times less than the radius of the atom.
Question. In the Gold foil experiment of Geiger and Marsden, that paved the way for Rutherford’s model of an atom, ~ 1.00% of the α-particles were found to deflect at angles > 500. If one mole of α-particles were bombarded on the gold foil, compute the number of α-particles that would deflect at angles less than 500.
Answer. % of α-particles deflected more than 500 =1% of α-particles. % of α-particles deflected less than 500 =100–1 = 99% Number of α-particles bombarded = 1 mole 23 = 6.022×1023 particles Number of particles that deflected at an angles less than 500




