Students can read the important questions given below for The d and f Block Elements Class 12 Chemistry. All The d and f Block Elements Class 12 Notes and questions with solutions have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS. You should read all notes provided by us and Class 12 Chemistry Important Questions provid ed for all chapters to get better marks in examinations. Chemistry Question Bank Class 12 is available on our website for free download in PDF.
Important Questions of The d and f Block Elements Class 12
Very Short Answer Questions
Question. Why is the 3rd ionization energy of Mn (Z = 25) is unexpectedly high ?
Answer. Due to half-filled electronic configuration.
Question. Why do transition metal (elements) show variable oxidation states ?
Answer. Due to presence of vacant d-orbitals.
Question. Write any uses of pyrophoric alloy.
Answer. Making bullets, shells and ligher flints.
Question. Define alloy.
Answer. Alloys are homogeneous solid solutions of two or more metals.
Question. Which element among 3d series exhibit only one oxidation state ?
Answer. SC
Question. Transition metals show zero oxidation state with ligands like CO. Explain.
Answer. Co form synergic bonding with metal ion.
Question. Arrange the given in increasing order of acidic character :
CrO3, CrO, Cr2O3.
Answer. CrO < Cr2O3 < CrO3
Question. Why KMnO4 or MnO4− ion is coloured ?
Answer. Due to charge transfer complex formation.
Question. Why can’t HCl acid be used to acidify KMnO4 solution ?
Answer. Because KMnO4 oxidize HCl into Cl2.
Question. Explain CuSO4.5H2O is blue while CuSO4 is colourless ?
Answer. Because water molecules act as ligands and results in crystal field splitting of d-orbitals of Cu2+ ion.
Question. Which element among 3d series exhibit highest oxidation state ?
Answer. Mn
Question. Name one ore of Mn and Cr.
Answer. Mn : MnO2
Cr : FeCr2O4
Question. Why Mn2+ compounds are more stable than Fe2+ compounds towards oxidation to their + 3 state ?
Answer. Mn+2 has half-filled electronic configuration.
Question. Which is more basic – La(OH)3 or Lu(OH)3 ? Why ?
Answer. La(OH)3, due to lanthanide contraction, lower size, more covalent character,least basic.
Question. In 3d series (Sc to Zn), the enthalpy of atomization of Zn is low. Why ?
Answer. Due to absence of unpaired electrons.
Question. Why is Ce4+ in aqueous solution a good oxidizing agent ?
Answer. Because Ce4+ is most stable in Ce+3 state in aqueous solution.
Question. Why do Zr and Hf exhibit similar properties ?
Answer. Due to lanthanide contraction.
Question. How would you account for the following :
Transition metals form coloured compounds?
Answer. Due to presence of vacant d-orbitals and d-d transitions, compounds of the transition metals are generally coloured. When an electron from a lower energy d-orbital is excited to a higher energy d-orbital, the energy of excitation corresponds to the frequency which generally lies in the visible region. The colour observed corresponds to the complementary colour of the light absorbed. The frequency of the light absorbed is determined by the nature of the ligand.
Question. Zn2+ salts are white while Cu2+ salts are coloured. Why?
Answer. Zn2+ ion has completely filled d-subshell and no d-d transition is possible. So zinc salts are white. Configuration of Cu2+ is [Ar] 3d9. It has partly filled d-subshell and hence it is coloured due to d-d transition.
Question. Why do transition elements show variable oxidation states?
Answer. Transition elements can use their ns and (n – 1)d orbital electrons for bond formation therefore, they show variable oxidation states.
For example – Sc has ns2(n – 1)d1 electronic configuration. It utilizes two electrons from its ns subshell then its oxidation state = +2. When it utilizes both the electrons then its oxidation state = +3.
Question. Name a member of the lanthanoid series which is well known to exhibit +4 oxidation state.
Answer. Lanthanoids showing +4 oxidation state are
58Ce, 59Pr, 65Tb and 66Dy.
Question. Assign reason for the following :
Copper (I) ion is not known in aqueous solution.
Answer. In aqueous solutions, Cu+ undergoes disproportionation to form a more stable Cu2+ ion.
2Cu+(aq) → Cu2+(aq) + Cu(s)Cu2+ in aqueous solutions is more stable than Cu+ ion because hydration enthalpy of Cu2+ is higher than that of Cu+. It compensates the second ionisation enthalpy of Cu involved in the formation of Cu2+ions.
Question. Explain giving reasons :
Transition metals and their compounds generally exhibit a paramagnetic behaviour.
Answer. Transition metals and most of their compounds contain unpaired electrons in the (n – 1)d orbitals hence show paramagnetic behaviour.
Question. Why Cd2+ salts are white?
Answer. It has completely filled d-orbital (d10).
Question. What is meant by ‘lanthanoid contraction’?
Answer. The steady decrease in the atomic and ionic radii (having the same charge) with increase in atomic number across the series from lanthanum to lutetium is known as lanthanoid contraction.
Question. Explain the following observations :
La3+ (Z = 57) and Lu3+ (Z = 71) do not show any colour in solutions.
Answer. Because they have empty 4f subshell.
Question. What are the different oxidation states exhibited by the lanthanoids?
Answer. Lanthanum and all the lanthanoids predominantly show +3 oxidation state. However, some of the lanthanoids also show +2 and +4 oxidation states in solution or in solid compounds. This irregularity arises mainly due to attainment of stable empty (4f0), half-filled (4f7) and fully filled (4f14) sub shell.
e.g. Ce4+ : 4f0 , Eu2+ : 4f7
Tb4+ : 4f7 , Yb2+ : 4f14
Question. Give reasons for the following :
Actinoids exhibit a greater range of oxidation
Answer. Actinoids exhibit greater range of oxidation states than lanthanoids. This is because there is less energy difference between 5f and 6d orbitals in actinoids than the energy difference between 4f and 5d orbitals in case of lanthanoids.
Question. Assign reasons for the following :
From element to element actinoid contraction is greater than the lanthanoid contraction.
Answer. The actinoid contraction is more than lanthanoid contraction because of poor shielding by 5f-electrons.
Question. Explain giving reasons :
The chemistry of actinoids is not as smooth as that of lanthanoids.
Answer. The chemistry of actinoids is not as smooth as lanthanoid because they show greater number of oxidation states due to comparable energies of 5f, 6d and 7s orbitals.
Question. Give reason :
There is a gradual decrease in the size of atoms with increasing atomic number in the series of lanthanoids.
Answer. As the atomic number increases, each succeeding element contains one more electron in the 4f orbital and one extra proton in the nucleus. The 4f electrons are rather inefiective in screening the outer electrons from the nucleus. As a result, there is gradual increase in the nuclear attraction for the outer electrons. Consequently, the atomic size gradually decreases. This is called lanthanoid contraction.
Short Answer Questions
Question. Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only ?
Answer. Oxygen and fluoride have small size and high electronegativity. They can oxidise the metal.
Question. Most of the transition metals do not displace hydrogen from dilute acids, why ?
Answer. Due to their –ve reduction potential.
Question. Explain why Cu+ is not stable in aqueous solution ?
Answer. Due to less –ve ΔhydHθ of Cu+/it cannot compensate 2nd ionization potential of Cu.
Question. Chromium is typical hard metal while mercury is a liquid. Explain why ?
Answer. Cr has five unpaired d-electrons. Hence metallic bonds are strong. In Hg, there is absence of unpaired electrons and size is larger.
Question. Write electronic configuration of Cu+2 and Co+2.
Answer. Cu+2 = [Ar] 3d9 4s0
Co+2 = [Ar] 3d7
Question. Balance the following equations :
(a) MnO4− + Fe2+ + H+ →
(b) Cr2O72− + Sn2+ + H+ →
Answer. (a) MnO4− + Fe2+ + H+ → Mn+2 + Fe+3
(b) Cr2O72− + Sn2+ + H+ → Cr+3 + Sn+4
Question. The following two reactions of HNO3 with Zn are given :
(a) Zn + conc. HNO3 → Zn(NO3)2 + X + H2O
(b) Zn + dil. HNO3 → Zn(NO3)2 + Y + H2O
Identify X and Y.
Answer. X = NO2
Y = N2O
Question. Sc, the first member of first transition series does not exhibit variable oxidation state. Why ?
Answer. Due to noble gas electronic configuration in + 3 oxidation state no other oxidation state is stable.
Question. Which of the following is/are transition element and why ?
Zn, Cd, Ag, Fe, Ni
Answer. Fe, Ni, Ag
Question. What are interstitial compounds ? Give example.
Answer. When small atoms like C, H, B and N occupy interstitial site in their lattice.
Example, TiC, Fe3H,
Question. Explain ‘Misch metal’ and write its use.
Answer. It is an alloy of 95% lanthanoid and 5% iron and traces of S, C, Ca and Al. Used in lighter flint, bullet tips etc.
Question.Why Zn, Cd, Hg are soft and have low melting point ?
Answer. Due to weak interatomic attraction/absence of unpaired electrons.
Question. Why do transition elements show variable oxidation states? In 3d series (Sc to Zn), which element shows the maximum number of oxidation states and why?
Answer. Variation in oxidation state : Transition elements can use their ns and (n – 1)d orbital electrons for bond formation. Therefore, they show variable oxidation state.
For example – Sc has ns2 (n – 1)d1 electronic configuration.
It utilizes two electrons from its ns subshell thenits oxidation state = +2. When it utilizes both the electrons then its oxidation state = +3.
Among the 3d series manganese (Mn) exhibits the largest number of oxidation states from +2 to +7 because it has maximum number of unparied electrons.
Mn – [Ar] 3d5 4s2
Question. What is meant by ‘disproportionation? Give an example of a disproportionation reaction in aqueous solution.
Answer. Disproportionation reaction involves the oxidation and reduction of the same substance. The
two examples of disproportionation reaction are :
(i) Aqueous NH3 when treated with Hg2Cl2 (solid) forms mercury aminochloride disproportionatively.
Hg2Cl2 + 2NH3 → Hg + Hg(NH2)Cl + NH4Cl
(ii) 2Cu+ → Cu + Cu2+
Question. Account for the following :
(i) Mn2+ is more stable than Fe2+ towards oxidation to +3 state.
(ii) The enthalpy of atomization is lowest for Zn in 3d series of the transition elements.
Answer. (i) Electronic configuration of Mn2+ is 3d5 which is half filled and hence stable. Therefore, third ionization enthalpy is very high, i.e., 3rd electron cannot be lost easily. In case of Fe2+, electronic configuration is 3d6. Hence it can lose one electron easily to give the stable configuration 3d5.
(ii) Zinc (Z = 30) has completely filled d-orbital (3d10) d-orbitals do not take part in interatomic bonding. Hence, metallic bonding is weak.
This is why it has very low enthalpy of atomisation (126 kJ mol–1).
Question. When chromite ore FeCr2O4 is fused with NaOH in presence of air, a yellow coloured compound (A) is obtained which on acidification with dilute sulphuric acid gives a compound (B) Compound (B) on reaction with KCl forms a orange coloured crystalline compound (C).
(i) Write the formulae of the compounds (A),
(B) and (C).
(ii) Write one use of compound (C).
Answer.

(ii) Potassium dichromate is used as a powerful oxidising agent in industries and for staining and tanning of leather.
Question. Complete the following equations :
(i) Cr2O2–7 + 2OH–
(ii) MnO–4 + 4H+ + 3e–
Answer. (i) Cr2O2–7 + 2OH– → 2CrO2–4 + H2O
(ii) MnO–4 + 4H+ + 3e– → MnO2 + 2H2O
Question. Complete the following equations :
(i) 2MnO4– + 5S2– + 16H+ →
(ii) Cr2O72– + 2OH– →
Answer. (i) H2S → 2H+ + S2–
5S2– + 2MnO–4 + 16H+ → 2Mn2+ + 8H2O + 5S
(ii) Cr2O2–7 + 2OH– → 2CrO2–4 + H2O
Question. Complete the following chemical equations :
(i) Fe3+ + I– →
(ii) CrO42– + H+ →
Answer. (i) 2Fe3+ + 2I– → 2Fe2+ + I2
(ii) 2CrO2–4 + 2H+ → Cr2O2–7 + H2O
Question. Describe the reactions involved in the preparation of K2Cr2O7 from chromite ore.
Answer. 4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 +2Fe2O3 + 8CO2
2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
Potassium dichromate is converted to chromate if pH is increased

Question. Complete the following chemical equations :
(i) 8MnO–4 + 3S2O32– + H2O →
(ii) Cr2O72– + 3Sn2+ + 14H+ →
Answer. (i) 8MnO–4 (aq) + 3S2O2–3(aq) + H2O(l) → 8MnO2(aq) + 6SO2–4(aq) + 2OH–(aq)
(ii) Cr2O2–7 + 3Sn2+ + 14H+ → 2Cr3+ + 3Sn4+ + 7H2O
Question. Complete the following equations
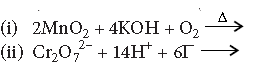
Answer.

Question. Assign a reason for each of the following observations :
(i) The transition metals (with the exception of Zn, Cd and Hg) are hard and have high melting and boiling points.
(ii) The ionisation enthalpies (first and second) in the first series of the transition elements are found to vary irregularly.
Answer. (i) As we move along transition metal series from left to right (i.e. Ti to Cu), the atomic radii decrease due to increase in nuclear charge. Hence the atomic volume decreases. At the same time, atomic mass increases. Hence, the density from titanium (Ti) to copper (Cu) increases.
(ii) Irregular variation of ionisation enthalpies is mainly attributed to varying degree of stability of different 3d-configurations (e.g., d0, d5, d10 are exceptionally stable).
Question. (a) Why is separation of lanthanoid elements difficult ?
(b) Transition metal exhibit higher enthalpies of atomization. Explain why ?
(c) Why have the transition metal high enthalpy of hydration ?
Answer. (a) Due to lanthanide contraction, the size of these elements is nearly same.
(b) Transition metal contain large number of unpaired electrons, and they have strong interatomic attractions.
(c) Due to their small size and large nuclear charge.
Question. (a) Deduce the number of 3d electrons in the following ions :
Cu2+, Sc+3
(b) Why do transition metals form alloy ?
(c) Why Zn+2 salts are white ?
Answer. (a) Cu+2 : 9 electrons
Sc+3 : 0 electron
(b) Transition metals have similar atomic radii.
(c) Absence of unpaired electron.
Long Answer Questions
Question.Compare the chemistry of the actinoids with that of lanthanoids with reference to
(i) electronic configuration
(ii) oxidation states
(iii) chemical reactivity.
Answer. (i) Electronic configuration : the general electronic configuration of lanthanoids is [Xe] 4f1–14 5d0–1 6s2 where as that of actinoids is [Rn] 5f1–14 6d0–1 7s2. Thus, lanthanoids involve the filling of 4f-orbitals whereas actinoids involve the filling of 5f-orbitals.
(ii) Oxidation states : Lanthanoids have principal oxidation state of +3. In addition, the lanthanoids show limited oxidation states such as +2, +3 and +4 because of large energy gap between 4f and 5d subshells. On the other hand, actinoids show a large number of oxidation states because of small energy gap between 5f and 6d subshells.
(iii) Chemical reactivity : (a) First few members of lanthanoids are quite reactive almost like calcium, where as actinoids are highly reactive metals especialy in the finely divided state.
(b) Lanthanoids react with dilute acids to liberate H2 gas whereas actinoids react with boiling water to give a mixture of oxide and hydride.
Question. A violet compound of manganes (A) decomposes on heating to liberate oxygen and compounds (B) and (C) of manganese are formed. Compound
(C) reacts with KOH in the presence of KNO3 to give compound (B). On heating compound (C) with conc. H2SO4 and NaCl, Cl2 gas is liberated and compound (D) of manganese is formed. Identify A, B, C, D alongwith reactions involved.
Answer.
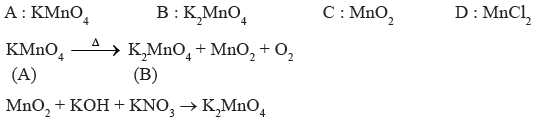
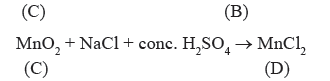
Question. (a) What is meant by disproportionation of an oxidation state ? Give one example.
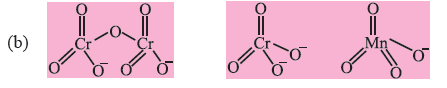
(b) Draw the structures of Cr2O72−, CrO4−2, MnO4−.
(c) What is the effect of lanthoids contraction beyond lanthanoid ?
Answer. (a) When any atom or ion undergo oxidation and reduction simultaneously it is called disproportionation.
(c) Size of respective 4d and 5d series elements becomes comparable from fourth group onwards (e.g., Zr and Hf).
