Please refer to Class 10 Science Sample Paper Set f below provided with solutions. All Class 10 Science Sample Paper have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS. All questions have been provided with solutions.
Class 10 Science Sample Paper Set f
Time: 3 Hours Maximum Marks: 80
General Instructions:
(i) The question paper comprises four sections A, B, C and D. There are 36 questions in the question papers. All questions are compulsory.
(ii) Section–A – question no. 1 to 20 – all questions and parts thereof are of one mark each. These questions contain multiple choice questions (MCQs), very short answer questions and assertion – reason type questions. Answers to these should be given in one word or one sentence.
(iii) Section–B – question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should in the range of 30 to 50 words.
(iv) Section–C – question no. 27 to 33 are short answer type questions, carrying 3 marks each. Answers to these questions should in the range of 50 to 80 words.
(v) Section–D – question no. – 34 to 36 are long answer type questions carrying 5 marks each. Answer to these questions should be in the range of 80 to 120 words.
(vi) There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
(vii) Wherever necessary, neat and properly labeled diagrams should be drawn
SECTION-A
Question 1. Copper displaces which of the following metal from its salt solution- (1 Marks)
a. ZnSO4
b. FeSO4
c. Ag NO3
d. NiSO4
OR
Why Respiration is considered an exothermic reaction?
Answer. FeSO4.
OR
Carbohydrates are broken down into glucose it combines with O2 and provides energy.
Question 2. Why is hydrogen included in the activity series of metal? (1 Marks)
Answer. Hydrogen can lose electron to form positive ion (H+)
Question 3. Which statement about rusting is incorrect? (1 Marks)
a. Corrosion of metals is called rusting
b. Rust is hydrated iron (III) oxide,Fe2O3.xH2O
c. The air pollutant, Sulphur dioxide, speed up rusting.
d. Greasing is used to protect machinery parts from rusting
Answer. Most metals corrode but only the corrosion of iron and steel called rusting
Question 4. Which of the following statements are usually correct for carbon compounds? These (1 Marks)
(i) Are good conductors of electricity
(ii) Are poor conductors of electricity
(iii) Have strong forces of attraction between their molecules
(iv) Do not have strong forces of attraction between their molecules
a) (i) and (ii)
b) (ii) and (iii)
c) (i) and (iv)
d) (ii) and (iv)
Answer. Option (d) ii and iv
Question 5. A metal M belongs to 13th group in the Modern Periodic table. Write the valency of the metal. (1 Marks)
Answer. Valency = 3
Question 6. Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic table? (1 Marks)
(a) The elements become less metallic in nature.
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
Answer. Option (C) The atoms lose their electrons more easily.
Question 7. Why do the walls of trachea not collapse when there is less air in it? (1 Marks)
OR
What are the components of the transport system in highly organized plants?
Answer. Tracheal walls do not collapse because it is supported by ring s of cartilage.
OR
Xylem and Phloem.
Question 8. When white light enters a prism it gets split into constituent colors. Which of this color is most deviated and which color is least deviated? (1 Marks)
Answer. Most deviated- Violet, least deviated – Red.
Question 9. 6Ω and12Ω resistor are connected in the parallel, this combination is connected in series with a 10 V battery and 6 Ω resistor. What is the potential difference between the terminals of the 12 Ω resistors? (1 Marks)
(a) 10 V
(b) 16V
(c) 14 V
(d) 4 V
OR
Name a device that helps to maintain a potential difference across a conductor.
Answer. Option (d) 4 V-
OR
Cell or battery
Question 10. State Right-hand thumb rules. (1 Marks)
OR
What kind of magnetic field is produced by current carrying solenoid?
Answer. When you are holding a current carrying straight conductor in your right hand, the thumb shows the direction of current and the folded fingers shows the direction of magnetic field.
OR
The magnetic field produced by a current carrying solenoid is similar to the magnetic field produced by a bar magnet.
Question 11. Newspaper reports about pesticide levels in ready-made food items are often seen these days and some states have banned these products. (1 Marks)
(i) What do you think would be the source of pesticides in these food items?
(ii) It is said that the harmful chemicals enter in to the food chain and accumulated in the top level organism of the food chain. What do you call the phenomenon?
iii) Write any one method to reduce the intake of pesticide.
Answer. (i) Chemicals/pesticides used to protect crops from diseases and pests
(ii) Bio magnification/Biological magnification Buy organic and locally grown fruit and vegetables. /Wash fruits and vegetables before eating/Grow your own produce. /Use non-toxic methods for controlling insects in the home and garden.
(iii) (any one method)
For question numbers 12, 13 and 14, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
a) Both A and R are true, and R is correct explanation of the assertion.
b) Both A and R are true, but R is not the correct explanation of the assertion.
c) A is true, but R is false.
d) A is false, but R is true.
Question 12. Assertion: The sex of a child is determined by the mother. (1 Marks)
Reason: Humans have two types of sex chromosomes: XX and XY
Answer. d) A is false, but R is true.
Question 13. Assertion: If a graph is plotted between the potential difference and the current flowing, the graph is a straight line passing through the origin. (1 Marks)
Reason: The current is directly proportional to the potential difference.
Answer. a) Both A and R are true, and R is correct explanation of the assertion.
Question 14. Assertion: White light is dispersed into its seven-color components by a prism. (1 Marks)
Reason: Different colors of light bend through different angles with respect to the incident ray as they pass through a prism.
Answer. a) Both A and R are true, and R is correct explanation of the assertion.
| Answer the questions (15, 16, 17, 18, 19 and 20) that follow on the basis of your understanding of the following source and the related studied concepts. |
Question 15. Tobacco Smoking
Tobacco is smoked in cigarettes, cigars and pipes. Tobacco smoke contains many harmful substances. The most damaging substances are tar, nicotine and carbon monoxide. Tobacco smoke is inhaled into the lungs. Tar from the smoke is deposited in the lungs and this prevents the lungs from working properly.
(i) Which one of the following is a function of the lungs? (1 Marks)
A) To pump oxygenated blood to all parts of your body
B) To transfer some of the oxygen that you breathe to your blood
C) To purify your blood by reducing the carbon dioxide content to zero
D) To convert carbon dioxide molecules into oxygen molecules.
Answer. B) To transfer some of the oxygen that you breathe to your blood
(ii) Tobacco smoking increases the risk of getting lung cancer and some other diseases. The risk of getting the disease due to tobacco smoking is _____________ (1 Marks)
A) Bronchitis
B) HIV/AIDS
C) Chicken pox
D) Hepatitis
Answer. A) Bronchitis
(iii) Some people use nicotine patches to help them to give up smoking. The patches are put on skin and release nicotine into the blood. This helps to relieve cravings and withdrawal symptoms when people have stopped smoking. Various methods are used to influence people to stop smoking.
Which of the following method of reducing smoking is based on Technology? (1 Marks)
A) Increase cost of cigarettes
B) Produce nicotine patches to help make people give up cigarettes.
C) Ban smoking in public areas.
Answer. B) Produce nicotine patches to help make people give up cigarettes.
Question 16. Due to smoking the alveoli damaged and the surface area reduces which leads to reduction in the absorption of oxygen. Name the deficiency disorder caused due to the reduction in the absorption of oxygen. (1 Marks)
Answer. Anaemia
Question 17. Toshio likes to look at stars. However, he cannot observe stars very well at night because he lives in a large city. Last year Toshio visited the countryside where he observed a large number of stars that he cannot see when he is in the city. (1× 4 marks)
(i) Why can many more stars be observed in the countryside than in large cities?
A) The moon is brighter in cities and blocks out the light from many stars.
B) There is more dust to reflect light in country air than in city air.
C) The brightness of city lights makes many stars hard to see.
D) The air is warmer in cities due to heat emitted by cars, machinery and houses.
Answer. C) The brightness of city lights makes many stars hard to see.
(ii) Toshio uses a telescope with a large diameter lens in order to observe stars of low brightness. Why does using a telescope with a large diameter lens make it possible to observe stars of low brightness?
A) The larger the lens the more light is collected.
B) The larger the lens the more it magnifies.
C) Larger lenses allow more of the sky to be seen.
D) Larger lenses can detect the dark colours in stars.
Answer. A) The larger the lens the more light is collected.
(iii) Most simple refracting telescopes have two convex lenses. The objective forms a real, inverted image at (or just within) the focal plane of the eyepiece. This image serves as the object for the eyepiece. The eyepiece forms a virtual, inverted image that is magnified. When object moves closer to convex lens, the image formed by it shift
A) Away from the lens
B) Towards the lens
C) First towards and then away from the lens
D) First away and then towards the lens.
Answer. A) Away from the lens
(iv) The magnifying power of a telescope is the ratio of an object’s angular diameter to its naked eye diameter. This depends on the focal length of both lenses. Magnification = focal length of objective lens / focal length of eyepiece lens A small refracting telescope has an objective of focal length 100 cm. If the eyepiece has a focal length of 4.0 cm, what is the magnification of the telescope?
A) 0.25 cm
B) 25 cm
C) 2.5 cm
D) 250 cm
Answer. B) 25 cm
Question 18. Cloning refers to the process of developing an embryo with the DNA from an adult animal. The newly created embryo is then zapped with electricity so that it starts multiplying, until it becomes a blastocyst (a small clump of cells that forms after an egg is fertilized), which is then implanted into a surrogate mother. (1× 4 marks)
(i) Cloning is a mode of ______________ reproduction. (Sexual/Asexual)
Answer. Asexual mode.
(ii) Clones are superior breeding animals used to produce healthier offspring. Animal cloning offers great benefits to consumers, farmers, and endangered species: Cloning allows farmers and ranchers to accelerate the reproduction of their most productive livestock in order to better produce safe and healthy food. An endangered species is a species that is very likely to become extinct in the near future, may be at risk due to factors such as habitat loss, poaching and invasive species. State the above statement is true or false.
Answer. True
(iii) Many organisms produce clones through the cloning process, which are genetically identical to the organisms from which they are derived without variations. What are variations?
Answer. The errors that take place during DNA copying.
(iv) Cloning also used in Horticulture refers to descendants of a single plant which were produced by vegetative reproduction. Name a plant that propagates through leaves.
Answer. Bryophyllum
Question 19. a). A current carrying conductor is placed perpendicular in the magnetic field, Name the rule which can be used to find the direction of force acting on the conductor.
b) State two ways to increase the force on a current-carrying conductor in magnetic field.
Answer. a. Fleming’s left hand rule
b. increasing the current, and using strong magnet
Question 20. Relative strength of magnetic field at a point in the space surrounding the magnet is shown by the (1 Marks)
a) Length of the magnet
b) Thickness of magnet
c) Degree of closeness of the field
d) Resistance offered by the surroundings.
Answer. c) Degree of closeness of the field
SECTION B
Question 21. Identify the type of chemical reaction taking place in each of the following; (2 marks)
a) Barium chloride solution is mixed with copper sulphate solution and a white precipitate is observed.
b) On heating copper powder in air in a china dish, the surface of copper turns black.
Answer. a) Double displacement /Precipitation reaction
b) Redox reaction/ Oxidation reaction
Question 22. Why some metal surfaces acquire a dull appearance when they are exposed to moist air? Write color acquired by the surfaces of copper and silver in such situation and also write the chemical names of the substances due to which it happens. (2 marks)
Answer. a) formation of layer of oxide, carbonate or sulphide
b) green layer copper carbonate, blackish tinge silver sulphide.
Question 23. a) What are Homologous series? (2 marks)
b) Why there is a gradation in physical properties of compounds in a Homologous series?
OR
The number of carbon compounds is more than those formed by all other elements put together. Justify the statement by giving two reasons.
Answer. a) A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series.
b) There is a gradation in physical properties, because the melting and boiling points increase with increasing molecular mass.
OR
i) Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large number of molecules –property of Catenation.
ii) Carbon has valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other element property of Tetravalency
Question 24. i) What is the mode of nutrition in Amoeba? (2 marks)
ii) Draw neat labelled diagram of nutrition in amoeba.
OR
Write any two differences between Aerobic and Anaerobic respiration.
Answer. i) Heterotrophic nutrition/ Holozoic nutrition
ii) Diagram with labelling – pg. 98 of NCERT science text book
OR
Aerobic- Take place in the presence of oxygen, End products are CO2 and H2O, Complete oxidation of food, more energy is produced. (any two points)
Anaerobic- Take place in the absence of oxygen, End products are CO2, alcohol, lactic acid, Incomplete oxidation of food, less energy is produced. (any two points)
Question 25. a) What determines the rate at which energy is delivered by a current? (2 marks)
b) Show how you would connect three resistors, each of resistance 6Ω, so that the combination has a resistance of 9Ω.
Answer. a) Power
b) One resistor in series and two resistors in parallel.
Question 26. What are the problems caused by the non-biodegradable wastes that we generate? (2 marks)
Answer. Causes Pollution of the environment – soil, water and air/ Spreading of diseases/Causing damage to soil microorganisms /Polluting the ground water. Any other valid points. (any two)
SECTION C
Question 27. a) Write the balanced chemical equations for the following reactions: (3 marks)
i) Calcium hydroxide + carbon di-oxide — Calcium carbonate +water
ii) Zinc + silver nitrate — Zinc nitrate + silver
b) Define Rancidity.
Answer. a) i) Ca (OH)2 + CO2 –→ CaCO3 + H2O
ii) Zn + 2AgNO3 –→ Zn (NO3)2 + 2Ag
b) The oxidation of fat containing food items is called rancidity.
Question 28. Give reasons: (3 marks)
a) Platinum, gold and silver are used to make jewellery.
b) Sodium, potassium and lithium are stored under oil.
c) Copper is used to make hot water tanks and not steel (an alloy of iron)
Answer. a) They are highly malleable and ductile (only ductile also can be considered)
b) They are highly reactive
c) Copper is a best/good conductor of heat than steel.
Question 29. i) How does the atomic radius change as you go (3 marks)
a) From left to right in a period?
b) Down a group in the periodic table?
ii)Two elements X and Y have atomic numbers 12 and 16 respectively. Write the electronic configuration for these elements. To which period of the Modern Periodic table do these two elements belong?
Answer. i) a) Decreases
b) Increases
ii) Atomic number X=12 Electronic configuration = 2,8,2
Atomic number Y = 16 Electronic configurations = 2,8,6
They belong to third period, because they have three shells.
Question 30. a) Draw neat diagram of human respiratory system and label- (3 marks)
i) The tube made up of rings of cartilage,
ii) The part through which gases are exchanged,
iii) The thin tubes that divides from bronchi.
OR
What are the methods used by plants to get rid of excretory products?
Answer. a) Neat diagram – NCERT text book page 104.
i) Trachea,
ii) Alveoli,
iii) Bronchioles
OR
Excess water by Transpiration, store in dead cells, shedding of leaves, stored in cellular vacuoles, stored as resins and gum, excrete some wastes in to the soil. (any three)
Question 31. A pea plant with blue colour flower denoted by BB is cross-bred with a pea plant with white flower denoted by ww. (3 marks)
i) What is the expected colour of the flowers in their F1 progeny?
ii) What will be the percentage of plants bearing white flower in F2 generation, when the flowers F1 plants were selfed?
iii) State the expected ratio of the genotype BB and Bw in the F2 progeny.
Answer. i) Blue
ii) 25%
iii) BB:Bw = 1:2
Question 32. If the image formed by a mirror for all positions of the object placed in front of it is always erect and diminished, what type of mirror is it? Draw a ray diagram to justify your answer. Where and why do we generally use this type of mirror? (3 marks)
OR
a) Find the focal length of a lens of power -2.0 D. What type of lens is this?
b) Define Refractive Index.
Answer. It is a convex mirror
Ray diagram-

Used in vehicle as rear-view mirror, to obtain wider angle of view.
OR
a) F= 1/P= 1/-2.0 = – 0.5 m or -50 cm. It is a concave lens.
b) The ratio of velocity of light between two medium is called as refractive index.
Question 33. What is meant by advance Sunrise and delayed sunset. Draw a labelled diagram to explain. (3 marks)
Answer. The layers of air nearer to the earth are denser than those above it, and the light rays pass through successive the denser layers. The rays bent more and more towards the normal.
To the observer, these rays appear to come from horizon. It is for this reason the sun appears 2 minutes earlier before sunrise and appears 2 minutes later after sunset.
SECTION -D
Question 34. i) Write the formula and chemical name of Bleaching powder. (5 marks)
ii) Write the chemical equation for the manufacture of bleaching powder.
iii) Give any two uses of bleaching powder.
iv) Name the chemical compound which is used for softening hard water.
v) Why does distilled water not conduct electricity, whereas rain water does?
OR
Define water of crystallization. Give the chemical formula for two compounds as examples. How can it be proved that the water of crystallization makes a difference in the state and colour of the compounds?
Answer. CaOCl2 and Calcium oxy chloride
ii) Ca (OH)2 + Cl2 —→ CaOCl2 + H2O
iii) Used to disinfect water, bleaching cotton and linen in textile industry, as an oxidizing agent, bleaching wood pulp. (any two)
iv) Sodium carbonate or washing soda
v) Because distilled water has no ions, but rain water has ions.
OR
* Water of crystallization is the fixed number of water molecules present in one formula unit of a salt.
* Examples- CuSO4.5H2O Na2CO3.10H2O (or any other)
* Heat a few crystals of hydrated copper sulphate (blue color) in a dry boiling tube.
* Water droplets are seen in the boiling tube.
* Colour: The colour of copper sulphate changes to white.
* State: The blue crystals changes to white powder
Question 35. a) Name the human male reproductive organ that produces sperms an also secretes a hormone. Write the functions of the secreted hormone. (5 marks)
b) Name the parts of the human female reproductive system where
i) Fertilization takes place,
ii) Implantation of the fertilized egg occurs.
c) Explain how the embryo gets nourishment inside the mother’s body.
Answer. a) Testes, testosterone. Testosterone controls the development of secondary sexual characters.
b) i. Oviduct or fallopian tube.
ii. Uterus
c) After implantation, a disc-like special tissue called placenta develops between the uterus wall and the embryo, which helps in the exchange of nutrients, oxygen and waste products between the embryo and the mother.
Question 36. a) The values of current I flowing in a given resistor for the corresponding values of potential difference V across the resistor are given below: (5 marks)

Plot a graph between V and I and calculate the resistance of that resistor.
b) List any two factors responsible for the resistance of a conductor.
c) Give one reason for the alloys used extensively in heating devices.
OR
a) Draw a schematic diagram of a circuit consisting of a battery of 3 cells of 2V each, a combination of three resistors of 5Ω, 8Ω and 12Ω and a plug key and an ammeter, all connected in series.
b) Write any three advantages of connecting resistors in parallel.
Answer. a)
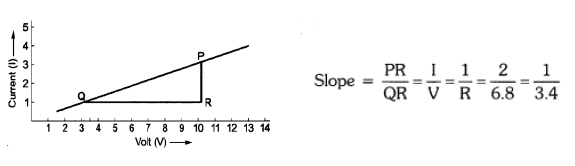
Thus, resistance, R = 3.4 Ω
b) Length of the conductor Rαl, area of cross section of the conductor Rα1/A, Nature of the material. (any two)
c) Alloys have higher resistivity, do not oxidize at higher temperatures, Very high melting point. (any two)
OR
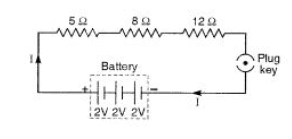
b) i) Parallel circuit divides the current through the electrical gadgets.
ii) The total resistance is decreased.
iii) Parallel circuits provide the same voltage to every source and appliance in the circuit, thus all appliances work efficiently.
iv) In a parallel circuit if one appliance is fused, the current continues to flow through the others. (any three)
We hoped you liked the Class 10 Science Sample Paper Set F given above. You can refer to more Class 10 science sample papers here. You should also refer to last year paper class 10 science
