Please refer to Classification of Elements and Periodicity in Properties Class 11 Chemistry notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Chemistry books for Class 11. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 11 Chemistry Classification of Elements and Periodicity in Properties Notes and Questions
Some Important Points and Terms of the Chapter
1. Dobereiner’s Triads:In 1817 a German chemist Doberneiner identified certain groups of three elements. These groups of three elements having similar properties was called triads. When three elements were arranged in order of their increasing atomic masses, the atomic mass of the middle element was roughly the mean of the atomic masses of the other two element
2. New Lands Law of octaves: When elements were arranged in order of their increasing relative atomic masses. The properties of every eight elements were similar to the first one, like the eighth note of a musical scale. This repetition in the properties of elements is just like the repetition of eighth node in an octave of music.
3. Mendeleev’s Periodic Law: The physical and chemical properties of elements are the periodic function of their atomic masses.
4. Mendeleev’s Periodic Table: When mendeleev started his work, 63 elements were known at that time. He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements. Mendeleev’s periodic table contains vertical columns called groups and horizontal rows called periods. There were 7 periods and 8 groups. Noble gases were not known
at that time. So there was no group of noble gases. The elements in each group of the periodic tables are similar to one another in many properties. The similar properties of the elements are repeated periodically
(a).Merits of mendeleev’s classification
• Mendeleev’s periodic law predicted the existence of some elements that had not been discovered at that time
• .Could predict the properties of several elements on the basis of their position in the periodic table.
• Could accommodate noble gases when they were discovered.
(b)Limitations of mendeleev’s classification :-
• The correct position could not be assigned to the hydrogen in the periodic table.
• Wrong order of the atomic masses of some elements could not be explained.
• The position of isotopes could not be explained.
• Uncertainty in prediction of new elements was there.
5. Modern periodic law: Properties of elements are the periodic function of their atomic number.
6. Modern Periodic Table: This table was prepared was Bohr and is based upon the electronic configuration of elements. The table consists of 18 vertical columns called groups Elements having similar outer electronic configurations in their atoms are arranged in vertical columns, referred to as groups . According to the recommendation of International Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18 and the table consists of 7 horizontal rows called periods. The first period contains 2 elements. The subsequent periods consists of 8, 8, 18, 18 and 32 elements, respectively. The seventh period is incomplete and like the sixth period would have a theoretical maximum (on the basis of quantum numbers) of 32 elements. In this form of the Periodic Table, 14 elements of both sixth and seventh periods (lanthanoids and actinoids, respectively) are placed in separate panels at the bottom
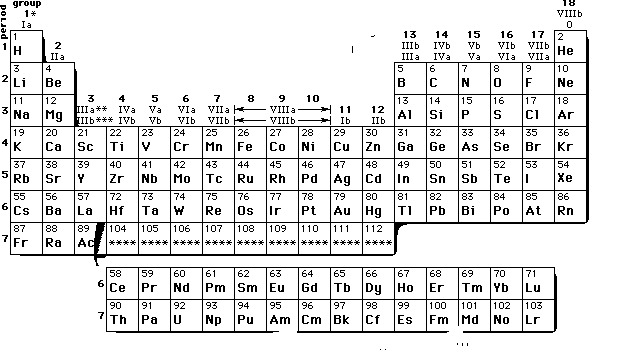
7. .Notation for IUPAC Nomenclature of Elements With Z > 100
8. We can classify the elements into four blocks viz., s-block, p-block, d-block and f-block depending on the type of atomic orbital that are being filled with electrons.
9. s-Block Elements :The elements of Group 1 (alkali metals) and Group 2 (alkaline earth metals) which have ns1and ns2 outermost electronic configuration belong to the s-Block Elements.
10. p-Block Elements The p-Block Elements comprise those belonging to Group 13 to 18 and these together with the s-Block Elements are called the Representative Elements or Main Group Elements. The outermost electronic configuration varies from ns2 np1 to ns2np6 in each period.
11. d-Block Elements These are the elements of Group 3 to 12 in the centre of the Periodic Table. These are characterised by the filling of inner d orbitals by electrons and are therefore referred to as d-Block Elements. These elements have the general outer electronic configuration (n-1)d1- 10ns0-2 .
12. f-Block Elements The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids, Ce(Z = 58) – Lu(Z = 71) and Actinoids, Th(Z = 90) – Lr (Z = 103) are characterised by the outer electronic configuration (n-2)f1-14 (n-1)d0–1ns2. The last electron added to each element is filled in f- orbital. These two series of elements are hence called the Inner- Transition Elements (f-Block Elements).
13. Variation in Atomic Radius in Period: The atomic size generally decreases across a period It is because within the period the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number increases resulting in the increased attraction of electrons to the nucleus.
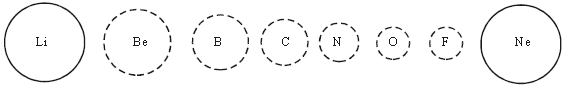
14. Variation in Atomic Radius in Group: Within a family or vertical column of the periodic table, the atomic radius increases regularly with atomic number as). as we descend the groups, the principal quantum number (n) increases and the valence electrons are farther from the nucleus. This happens because the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus. Consequently the size of the atom increases as reflected in the atomic radii.
15. The atomic radii of noble gases are not considered here. Being monatomic, their (non-bonded radii) values are very large. In fact radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of other elements.
16.A cation is smaller than its parent atom because it has fewer electrons while its nuclear charge remains the same. The size of an anion will be larger than that of the parent atom because the addition of one or more electrons would result in increased repulsion among the electrons and a decrease in effective nuclear charge. For example, the ionic radius of fluoride ion (F– ) is 136 pm whereas the atomic radius of fluorine is only 64 pm. On the other hand, the atomic radius of sodium is 186 pm compared to the ionic radius of 95 pm for Na+.
17. Isoelectronic species : Atoms and ions which contain the same number of electrons.. For example, O2–, F–, Na+ and Mg2+ have the same number of electrons (10). Their radii would be different because of their different nuclear charges. The cation with the greater positive charge will have a smaller radius because of the greater attraction of the electrons to the nucleus. Anion with the greater negative charge will have the larger radius. In this case, the net repulsion of the electrons will outweigh the nuclear charge and the ion will expand in size.
18. Ionization Enthalpy: It represents the energy required to remove an electron from an isolated gaseous atom (X) in its ground state. In other words, the first ionization enthalpy for an element X is the enthalpy change (ΔiH) for the reaction depicted in equation. X (g) → X+(g) + e– . The ionization enthalpy is expressed in units of kJ mol–1. We can define the second ionization enthalpy as the energy required to remove the second most loosely bound electron; it is the energy required to carry out the reaction shown in equation X+(g) → X2+(g) + e– . Energy is always required to remove electrons from an atom and hence ionization enthalpies are always positive. The second ionization enthalpy will be higher than the first ionization enthalpy because it is more difficult to remove an electron from a positively charged ion than from a neutral atom.In the same way the third ionization enthalpy will be higher than the second and so on. The term ―ionization enthalpy‖, if not qualified, is taken as the first ionization enthalpy.
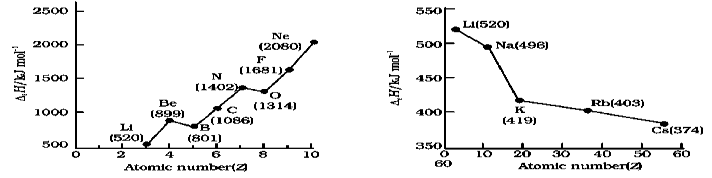
19. Variation in Ionization Enthalpy in Group: As we descend in a group the first ionization enthalpy generally decreases .Because as we go down a group, the outermost electron being increasingly farther from the nucleus, there is an increased shielding of the nuclear charge by the electrons in the inner levels. In this case, increase in shielding outweighs the increasing nuclear charge and the removal of the outermost electron requires less energy down a group.
20. Variation in Ionization Enthalpy in Period: The first ionization enthalpy generally increases as we go across a period. When we move from left to right in period, successive electrons are added to orbitals in the same principal quantum level and the shielding of the nuclear charge by the inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus. Thus, across a period, increasing nuclear charge outweighs the shielding. Consequently, the outermost electrons are held more and more tightly and the ionization enthalpy increases across a period
21. Electron Gain Enthalpy: When an electron is added to a neutral gaseous atom (X) to convert it into a negative ion, the enthalpy change accompanying the process is defined as the Electron Gain Enthalpy (ΔegH). Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion as represented by equation. X(g) + e– → X–(g) . Depending on the element, the process of adding an electron to the atom can be either endothermic or exothermic. For many elements energy is released when an electron is added to the atom and the electron gain enthalpy is negative. For example, group 17 elements (the halogens) have very high negative electron gain enthalpies because they can attain stable noble gas electronic configurations by picking up an electron. On the other hand, noble gases have large positive electron gain enthalpies because the electron has to enter the next higher principal quantum level leading to a very unstable electronic configuration.
22. Variation in electron gain enthalpies in Group & period: The variation in electron gain enthalpies of elements is less systematic than for ionization enthalpies. As a general rule, electron gain enthalpy becomes more negative with increase in the atomic number across a period. The effective nuclear charge increases from left to right across a period and consequently it will be easier to add an electron to a smaller atom since the added electron on an average would be closer to the positively charged nucleus. We should also expect electron gain enthalpy to become less negative as we go down a group because the size of the atom increases and the added electron would be farther from the nucleus. This is generally the case. However, electron gain enthalpy of O or F is less negative than that of the succeeding element. This is because when an electron is added to O or F, the added electron goes to the smaller n = 2 quantum level and suffers significant repulsion from the other electrons present in this level. For the n = 3 quantum level (S or Cl), the added electron occupies a larger region of space and the electron electron repulsion is much less.
23. Electronegativity: A qualitative measure of the ability of an atom in a chemical compound to attract shared electrons to itself is called electro negativity Linus Pauling, an American scientist, in 1922 assigned arbitrarily a value of 4.0 to fluorine, the element considered to have the greatest ability to attract electrons. Electronegativity generally increases across a period from left to right (say from lithium to fluorine) and decrease down a group (say from fluorine to astatine) in the periodic table.
24. Anomalous Properties of Second Period Elements: The first element of each of the groups 1 (lithium) and 2 (beryllium) and groups 13-17 (boron to fluorine) differs in many respects from the other members of their respective group. For example, lithium unlike other alkali metals, and beryllium unlike other alkaline earth metals, form compounds with pronounced covalent character; the other members of these groups predominantly form ionic compounds. In fact the behaviour of lithium and beryllium is more similar with the second element of the Group 1, 2 ,13, 14, 15, 16 ,17. following group i.e., magnesium and aluminum, respectively. This sort of similarity is commonly referred to as diagonal relationship in the periodic properties. The anomalous behaviour is attributed to their small size, large charge/ radius ratio and high electronegativity of the elements. In addition, the first member of group has only four valence orbitals (2s and 2p) available for bonding, whereas the second member of the groups have nine valence orbitals (3s, 3p, 3d). As a consequence of this, the maximum covalency of the first member of each group is 4 (e.g., boron can only form[BF4]– , whereas the other members of the groups can expand their valence shell to accommodate more than four pairs of electrons e.g., aluminum forms [AlF6]3- ). Furthermore, the first member of p-block elements displays greater ability to form pΠ – pΠ multiple bonds to itself (e.g., C = C, C ≡ C, N = N, N ≡ N) and to other second period elements (e.g., C = O, C = N, C ≡ N, N = O) compared to subsequent members of
the same group.
Question: What the s- and p- block elements are collectively called?
Ans: Representative elements.
Question: What the elements of a group have common among them?
Ans: They have same number of electrons in the valence shell.
Question: State the modern periodic law.
Ans: The physical and chemical properties of the elements are the periodic function of their atomic numbers.
Question: In how many groups and periods the elements in modern periodic table are classified?
Ans: In 18 groups and 7 periods.
Question: What do you mean by electronic configuration of the elements?
Ans: The systematic distribution of the electrons among the orbitals of an atom of an element according to increasing order of their energies is called as electronic configuration of that element.
Question: Name the groups of elements classified as s-, p- and d- blocks.
Ans: s- block= 1,2 (including He), p- block= 13 to 18 (except He), d- block= 3 to 12.
Question: Define atomic radius.
Ans: The one-half the distance between the nuclei of two covalently bonded atoms of the same element in a molecule is called as atomic radius.
Question: Select the species which are iso-electronic (same number of electron) with each other.
(1) Ne (2) Cl– (3) Ca2+ (4) Rb+
Ans: The Cl– and Ca2+. Both have 18 e_ each.
Question: Define the term ionisation enthalpy.
Ans: The energy required to remove the outer most electron from the valence shell of an isolated gaseous atom is called as ionisation enthalpy.
Question: Elements in the same group have equal valency. Comment on it.
Ans: Because the general outer most electronic configurations of the elements of a group remain same and they contain equal number of electrons in their respective outer most shells.
Question: Arrange the following in the order of increasing radii:
(a) I, I+, I– (b) F, Cl, Br
Ans: (a)I+ < I < I+ (b) O < N< P
Question: The outer electronic configuration of some elements are:
(a) 3s2 3p4 (b) 3d104s2 (c) 3s2 3p6 4s2 (d) 6s2 4f3
To which block of elements in the periodic table each of these belongs?
Ans: (a) p- Block (b) d- Block (c) s- Block (d) f- Block
Question: What is meant by periodicity in properties of elements? What is the reason behind this?
Ans: The repetition of similar properties after regular intervals is called as periodicity. It is due to the similarity in the outer electronic configurations which gives rise to the periodic properties of the elements.
Question: How do atomic radii vary in a group and a period?
Ans: In group- Atomic size increases on moving from top to bottom.
In period- Atomic size decreases on moving left to right in a period.
Question: Noble gases have zero electron gain enthalpy values. Explain.
Ans: Because the outer most shell of noble gases is completely filled and no more electrons can be added.
Question: Describe the two merits of long form periodic table over the Mendeleev’s periodic table?
Ans: 1. It removed the anomalies about the position of isotopes which existed in the Mendeleev’s table.
2. It relates the position of an element in the periodic table with its electronic configuration.
Question: What is a period in the periodic table? How do atomic sizes change in a period with an increase in atomic number?
Ans: The horizontal rows in periodic table are called as periods. The atomic sizes decrease in a period with an increase in atomic number.
Question: Name the factors which affect the ionisation enthalpy of an element.
Ans: (i) Size of atom or ion
(ii) Nuclear charge
(iii) Electronic configuration
(iv) Screening effect
(v) Penetration effect of the electrons
Question: How does ionisation enthalpy vary in a group and a period?
Ans: In Period- It increases from left to right
In group- It decreases down the group.
Question: Explain why are cations smaller and anions larger in size than their parent atoms?
Ans: (a) The cations are smaller than their parent atoms due to the following reasons:
(i) Disappearance of the valence shell.
(ii) Increase of effective nuclear charge
(b) The anions are larger than their parent atoms due to the following reason:
An increase in the number of electrons in the valence shell reduces the effective nuclear charge due to greater mutual shielding by the electrons. As a result, electron cloud expands, i.e., the ionic radius increases.
Question: Describe the theory associated with the radius of an atom as it
(a) gains an electron
(b) loses an electron
Ans: (a) When an atom gains an electron, its size increases. When an electron is added, the number of electrons goes up by one. This results in an increase in repulsion among the electrons. However, the number of protons remains the same. As a result, the effective nuclear charge of the atom decreases and the radius of the atom increases.
(b) When an atom loses an electron, the number of electrons decreases by one while the nuclear charge remains the same. Therefore, the interelectronic repulsions in the atom decrease. As a result, the effective nuclear charge increases. Hence, the radius of the atom decreases.
Question: Among the elements of the second period Li to Ne pick out the element:
(i) with the highest first ionisation energy
(ii) with the highest electronegativity
(iii) with the largest atomic radius Give the reason for your choice.
Ans: (i) The ionisation energy increases on going from left to right. Therefore, the element with the highest ionisation energy is Ne.
(ii) The electro negativity is electron- accepting tendency. This increases on going from left to right and decreases down the group. Therefore, the element with the highest electro- negativity is F.
(iii) The atomic radius decreases across a period on going from left to right. Thus, the first element of any period should have the largest atomic radii. Here, Li has the largest atomic radii.
Question: The first ionisation enthalpy of magnesium is higher than that of sodium. On the other hand, the second ionisation enthalpy of sodium is very much higher than that of magnesium. Explain.
Ans: The 1st ionisation enthalpy of magnesium is higher than that of Na due to higher nuclear charge and slightly smaller atomic radius of Mg than Na. After the loss of first electron, Na+ formed has the electronic configuration of neon (2,8). The higher stability of the completely filled noble gas configuration leads to very high second ionisation enthalpy for sodium. On the other hand, Mg+ formed after losing first electron still has one more electron in its outermost (3s) orbital. As a result, the second ionisation enthalpy of magnesium is much smaller than that of sodium.
Question: What are the major differences between metals and non- metals?
Ans:

Question: Arrange the following as stated:
(i) N2, O2, F2, Cl2 (Increasing order of bond dissociation energy)
(ii) F, Cl, Br, I (Increasing order of electron gain enthalpy)
(iii) F2, N2, Cl2 , O2(Increasing order of bond length)
Ans: (i) F2 < Cl2 < O2 < N2
(ii) I < Br < F < Cl
(iii) N2 < O2 < F2 < Cl2
Question: Why does the first ionisation enthalpy increase as we go from left to right through a given period of the periodic table?
Ans: In a period, the nuclear charge (the number of protons) increases on going from left to right. The electron added to each element from left to right enters the same shell. This results in an increase of the effective nuclear charge across the period on moving from left to right. As a result, the electron get more firmly bound to the nucleus. This causes an increase in the first ionisation enthalpy across the period.
Question: How does atomic radius vary in a period and in a group? How do you explain the variation?
Ans: Atomic radius generally decreases from left to right across a period. This is because within a period, the outer electrons are present in the same valence shell and the atomic number increases from left to right across a period, resulting in an increased effective nuclear charge. As a result, the attraction of electrons to the nucleus increases.
On the other hand, the atomic radius generally increases down a group. This is because down a group, the principal quantum number (n) increases which results in an increase of the distance between the nucleus and valence electrons.
Question: Consider the following species:
N3–, O2–, F–, Na+, Mg2+ and Al3+
(a) What is common in them?
(b) Arrange them in the order of increasing ionic radii.
Ans: (a) the same number of electrons (10 electrons). Hence, the given species are isoelectronic.
(b) Al3+ < Mg2+ < Na+ < F–< O2– < N3–
Question: Use the periodic table to answer the following questions.
(i) Identify the element with five electrons in the outer sub-shell.
(ii) Identify an element that would tend to lose two electrons.
(iii) Identify an element that would tend to gain two electrons.
Ans: (i) Chlorine
(ii) Magnesium
(iii) Oxygen
Question: The first (ΔiH1) and the second (ΔiH) ionization enthalpies (in kJ mol–1) and the (ΔegH) electron gain enthalpy (in kJ mol–1) of a few elements are given below:

Which of the above elements is likely to be :
(a) the least reactive element.
(b) the most reactive metal.
(c) the most reactive non-metal.
(d) the least reactive non-metal.
(e) the metal which can form a stable binary halide of the formula MX2, (X=halogen).
(f) the metal which can form a predominantly stable covalent halide of the formula MX (X=halogen)?
Ans: (a) Element V is likely to be the least reactive element. This is because it has the highest first ionization enthalpy (ΔiH1) and a positive electron gain enthalpy (ΔegH).
(b) Element II is likely to be the most reactive metal as it has the lowest first ionization enthalpy (ΔiH1) and a low negative electron gain enthalpy (ΔegH).
(c) Element III is likely to be the most reactive non–metal as it has a high first ionization enthalpy (ΔiH1) and the highest negative electron gain enthalpy (ΔegH).
(d) Element V is likely to be the least reactive non–metal since it has a very high first ionization enthalpy (ΔiH2) and a positive electron gain enthalpy (ΔegH).
(e) Element VI has a low negative electron gain enthalpy (ΔegH). Thus, it is a metal. Further, it has the lowest second ionization enthalpy (ΔiH2). Hence, it can form a stable binary halide of the formula MX2(X=halogen).
(f) Element I has low first ionization energy and high second ionization energy. Therefore, it can form a predominantly stable covalent halide of the formula MX (X=halogen).
Question: What is the cause of the periodicity in the properties of the elements? How do the following properties vary in (a) a group and (b)in a period
(i) electronegativity
(ii) ionisation enthalpy
(iii) Atomic size
Ans: It is due to the similarity in the outer electronic configurations which gives rise to the periodic properties of the elements.
(a) In a group:
(i) Electronegativity- It decreases down the group.
(ii) Ionisation enthalpy- It decreases down the group.
(iii) Atomic size- It increases down the group.
(b) In a period:
(i) Electronegativity- Increases
(ii) Ionisation enthalpy- Increases
(iii) Atomic size- Dereases.



