Check the below NCERT Get Amines MCQ Questions for Class 12 with Answers available with PDF free download. MCQ Questions for Class 12 Chemistry with Answers were prepared based on the latest syllabus and examination pattern issued by CBSE, NCERT and KVS. Our teachers have provided below Amines Class 12 Chemistry MCQs Questions with answers which will help students to revise and get more marks in exams
Get Amines MCQ Questions for Class 12 with Answers
Refer below for Get Amines MCQ Questions for Class 12 with solutions. Solve questions and compare with the answers provided below
Question. Which of the following reactions is appropriate for converting acetamide to methanamine?
(a) Hoffmann hypobromamide reaction
(b) Stephen’s reaction
(c) Gabriel phthalimide synthesis
(d) Carbylamine reaction
Answer
A
Question. Which of the following will be most stable diazonium salt RN2+X –?
(a) CH3N2+X–
(b) C6H5N2+X–
(c) CH3CH2N2+X–
(d) C6H5CH2N2+X–
Answer
B
Question. Aniline is reacted with bromine water and the resulting product is treated with an aqueous solution of sodium nitrite in presence of dilute hydrochloric acid. The compound so formed is converted into a tetrafluoroborate which is subsequently heated to dry. The final product is
(a) p-bromoaniline
(b) p-bromofluorobenzene
(c) 1, 3, 5-tribromobenzene
(d) 2, 4, 6-tribromofluorobenzene.
Answer
D
Question. Nitrobenzene on reaction with conc. HNO3/H2SO4 at 80–100°C forms which one of the following products?
(a) 1, 4-Dinitrobenzene
(b) 1, 2, 4-Trinitrobenzene
(c) 1, 2-Dinitrobenzene
(d) 1, 3-Dinitrobenzene
Answer
D
Question. Method by which aniline cannot be prepared is
(a) degradation of benzamide with bromine in alkaline solution
(b) reduction of nitrobenzene with H2/Pd in ethanol
(c) potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution
(d) hydrolysis of phenylisocyanide with acidic solution.
Answer
C
Question. The electrolytic reduction of nitrobenzene in strongly acidic medium produces
(a) azobenzene
(b) aniline
(c) p-aminophenol
(d) azoxybenzene.
Answer
C
Question. Acetamide is treated with the following reagents separately. Which one of these would yield methyl amine?
(a) NaOH-Br2
(b) Sodalime
(c) Hot conc.H2SO4
(d) PCl5
Answer
A
Question. Which one of the following on reduction with lithium aluminium hydride yields a secondary amine?
(a) Methyl isocyanide
(b) Acetamide
(c) Methyl cyanide
(d) Nitroethane
Answer
A
Question. Electrolytic reduction of nitrobenzene in weakly acidic medium gives
(a) N-phenylhydroxylamine
(b) nitrosobenzene
(c) aniline
(d) p-hydroxyaniline.
Answer
C
Question. The correct sequence of reactions to convert p-nitrophenol into quinol involves
(a) reduction, diazotisation and hydrolysis
(b) hydrolysis, diazotisation and reduction
(c) hydrolysis, reduction and diazotisation
(d) diazotisation, reduction and hydrolysis
Answer
A
Question.

(a) CH3Br
(b) CH3CONH2
(c) CH3NH2
(d) CHBr3
Answer
C
Question. In the chemical reaction,
CH3CH2NH2 + CHCI3 + 3KOH → A + B + 3H2O
The compounds A and B are respectively
(a) CH3CH2CONH2 and 3KCI
(b) C2H5NCand K2CO3
(c) C2H5NCand 3KCI
(d) C2H5CNand 3KCI
Answer
C
Question. Why do 2° and 3° amines fail to undergo the carbylamine test?
(a) They combine with chloroform to give a stable compound
(b) They react with alcoholic KOH
(c) The nitrogen atom of the amine group does not have the required number of hydrogen atoms
(d) All the given reasons are conect
Answer
B
Question. C2H5NH2 →HNO2 A →PCI B →NH3 C.
Recognize the compound C from the following
(a) propanenitrile
(b) methylamine
(c) ethylamine
(d) acetamide
Answer
C
Question. Consider the following reaction,
C6H5NO2 →Sn/HCI X →C6H5COCI Y + HCl What is Y ?
(a) Acetanilide
(b) Benzanilide
(c) Azobenzene
(d) Hydrazobenzene
Answer
B
Question. Which of the following is most basic in nature?
(a) NH3
(b) CH3NH2
(c) (CH3)2NH
(d) C6H5N(CH3)2
Answer
C
Question. Given the following sequence of reactions,

The major product C is
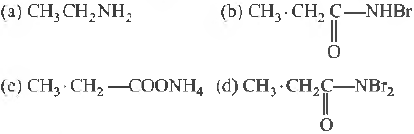
Answer
A
Question. Amino group is ortho,para-directing for aromatic electrophilic substitution. On nitration of aniline, a good amount of m-nitroaniline is obtained. This is due to
(a) in nitration mixture, ortho, para-activity of NH2 group is completely lost
(b) —NH2 becomes —NH+3, which is m-clirecting
(c) —NH2 becomes —NH+SO–4 , which is m-clirecting
(d) —NH2 becomes —NH–SO+2 , which ism-directing
Answer
A
Question. Choose the amide which on reduction with LiAIH4 yields a secondary amine
(a) ethanamide
(b) N-methylethanamide
(c) N, N-dimethylethanamide
(d) phenylmethanamide
(e) butanamide
Answer
B
Question. Name of method use to separate primary, secondary and tertiary amines is
(a) Hofmann method
(b) Lucas method
(c) Victor Meyer method
(d) Kolbe method
Answer
A
Question. Comparing basic strength of NH3 , CH3 NH2 and C6H5NH2 it may be concluded that
(a) basic strength remains unaffected
(b) basic strength of alkyl amines is lowest
(c) basic strength of aryl amines is lowest
(d) basic strength of NH3 is highest
Answer
C
Question. Which of the following is strongest base?
(a) C6H5NH2
(b) p -NO2—C6H4NH2
(c) m-NO2—C6HNH2
(d) C6H5CH2NH2
Answer
D
Question. Intermediates formed during reaction of RCONH2 with Br2 and KOH are
(a) RCONHBr and RNCO
(b) RNHCOBr and RNCO
(c) RNH – Br and RCONHBr
(d) RCONBr2
Answer
A
Question. Amides may be converted into amines by a reaction named after
(a) Hoffmann
(b) Claisen
(c) Perkin
(d) Kekule.
Answer
A
Question. Indicate which nitrogen compound amongst the following would undergo Hoffmann reaction (i.e.,reaction with Br2 and strong KOH) to furnish the primary amine (R–NH2)?
(a) RCONHCH3
(b) RCOONH4
(c) RCONH2
(d) R – CO – NHOH
Answer
C
Question. The correct order of the basic strength of methyl substituted amines in aqueous solution is
(a) CH3NH2 > (CH3)2NH > (CH3)3N
(b) (CH3)2NH > CH3NH2 > (CH3)3N
(c) (CH3)3N > CH3NH2 > (CH3)2NH
(d) (CH3)3N > (CH3)2NH > CH3NH2
Answer
B
Question. Nitration of aniline in strong acidic medium also gives m-nitroaniline because
(a) inspite of substituents nitro group always goes to only m-position
(b) in electrophilic substitution reactions amino group is meta directive
(c) in absence of substituents nitro group alwaysgoes to m-position
(d) in acidic (strong) medium aniline is present as anilinium ion.
Answer
D
Question. The correct statement regarding the basicity of arylamines is
(a) arylamines are generally more basic than alkylamines because of aryl group
(b) arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is sp-hybridised
(c) arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalised by interaction with the aromatic ring p-electron system
(d) arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalised by interaction with the aromatic ring p-electron system.
Answer
C
Question. Phenyl isocyanides are prepared by which of the following reaction?
(a) Reimer–Tiemann reaction
(b) Carbylamine reaction
(c) Rosenmund’s reaction
(d) Wurtz reaction
Answer
B
Question. The compound obtained by heating a mixture of ethylamine and chloroform with ethanolic potassium hydroxide (KOH) is
(a) an amide
(b) an amide and nitro compound
(c) an ethyl isocyanide
(d) an alkyl halide.
Answer
C
Question. The action of nitrous acid on an aliphatic primary amine gives
(a) secondary amine
(b) nitro alkane
(c) alcohol
(d) alkyl nitrite.
Answer
C
Question. Which one of the following order is wrong, with respect to the property indicated?
(a) Benzoic acid > phenol > cyclohexanol (acid strength)
(b) Aniline > cyclohexylamine > benzamide (basic strength)
(c) Formic acid > acetic acid > propanoic acid (acid strength)
(d) Fluoroacetic acid > chloroacetic acid >bromoacetic acid (acid strength)
Answer
B
Question. For carbylamine reaction, we need hot alcoholic KOH and
(a) any primary amine and chloroform
(b) chloroform and silver powder
(c) a primary amine and an alkyl halide
(d) a monoalkylamine and trichloromethane.
Answer
A
Question. Which product is formed, when acetonitrile is hydrolysed partially with cold concentrated HCl?
(a) Methyl cyanide
(b) Acetic anhydride
(c) Acetic acid
(d) Acetamide
Answer
D
Question. On hydrolysis of a “compound”, two compounds are obtained. One of which on treatment with sodium nitrite and hydrochloric acid gives a product which does not respond to iodoform test. The second one reduces Tollens’ reagent and Fehling’s solution. The “compound” is
(a) CH3CH2CH2NC
(b) CH3CH2CH2CN
(c) CH3CH2CH2ON O
(d) CH3CH2CH2CON(CH3)2
Answer
A
Question. Which of the following statements about primary amines is false?
(a) Alkyl amines are stronger bases than aryl amines.
(b) Alkyl amines react with nitrous acid to produce alcohols.
(c) Aryl amines react with nitrous acid to produce phenols.
(d) Alkyl amines are stronger bases than ammonia.
Answer
C
Question. Which of the following is more basic than aniline?
(a) Benzylamine
(b) Diphenylamine
(c) Triphenylamine
(d) p-Nitroaniline
Answer
A

We hope you liked Get Amines MCQ Questions for Class 12 with answers provided above. In case you have any questions please post them in the comments section below and our Chemistry teachers will provide a response.
