Please refer to Metals and Non-Metals Class 10 Science Important Questions given below. These solved questions for Metals and Non-Metals have been prepared based on the latest CBSE, NCERT and KVS syllabus and books issued for the current academic year. We have provided important examination questions for Class 10 Science all chapters.
Class 10 Science Metals and Non-Metals Important Questions
Very Short Answer Type Questions
Question. Name the metals Which have low melting point.
Answer : Gallium and Caesium.
Question. Name a non-metal which is lustrous whereas a metal which is non-lustrous.
Answer : Iodine is a lustrous non-metal, Lead is a non-lustrous metal.
Question. Choose the amphoteric oxide amongst the following:
Na2O, ZnO, Al2O3, CO2, H2O
Answer : ZnO and Al2O3 are amphoteric oxides.
Question. What happens when carbon dioxide is compressed in water at high pressure?
Answer : CO2 + H2O → H2CO3 Carbonic acid is formed.
Question. Why do we apply paint on iron grills?
Answer : Iron grills are painted to prevent them from rusting.
Question. Why does stainless steel not get rusted easily?
Answer : Stainless steel is an alloy of Fe, C, Cr and Ni, therefore it does not get rusted.
Question. Why do we use gold and platinum metals in jewellery?
Answer : Gold and Platinum are lustrous metals and do hot react with substances present in the atmosphere, therefore remain lustrous for a long time.
Question. Why oxides of highly reactive metals cannot be reduced by carbon?
Answer : It is because highly reactive metals themselves are good reducing agents, so they can’t be reduced by carbon.
Question. An element ‘A’ form two oxides AO and AO2. The oxide AO is neutral whereas the oxide AO2 is acidic in nature. Would you call element ‘A’ a metal or a non-metal?
Answer : The element is carbon which is a non- metal. CO is neutral and CO2 is acidic in nature.
Question. Why do silver ornaments lose their shine when kept in open air for sometime?
Answer : It is because Ag reacts with H2S present in the atmosphere to form Ag2S due to which it loses its shine.
Question. Why do we use copper and aluminium wire for transmission of electric current?
Answer : Copper and aluminium are ductile and good conductors of electricity, therefore they are used in transmission wires.
Question. Give reason why: Electric wires are coated with plastic.
Answer : Plastic is a non-conductor of electricity, therefore electric wires are coated with plastic.
Question. Arrange the following metals in decreasing order of reactivity are Na, K, Cu, Ag
Answer : K > Na > Cu > Ag is the decreasing order of reactivity.
Question. Which of the following two metals will melt at body temperature (37°C): Gallium, Magnesium, Caesium, Aluminium?
Answer : Gallium and Caesium will melt at the body temperature.
Question. Show the electronic transfer in formation of MgCl2 from its elements.
Answer :
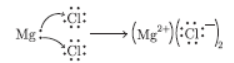
Question. Give composition of the alloy: brass and bronze.
Answer : Brass is made up of Cu and Zn. Bronze is made up of Cu and Sn.
Question. What is the formula of rust?
Answer : Rust is hydrated ferric oxide, Fe2O3 xH2O.
Question. An alloy has low melting point and is therefore used for electrical fuse. Name the alloy and write its constituents.
Answer : Solder is an alloy. It is made up lead and tin.
Question. Name one metal and one non-metal in liquid state at room temperature.
Answer : Mercury is a metal and Bromine is a non-metal present as a liquid at room temperature.
Question. Name two metals which react with dilute HNO3 to evolve H2 gas.
Answer : Mn and Mg.
Short Answer Type Questions :
Question. (a) Name a metal for each case :
(i) It does not react with cold as well as hot water but reacts with steam.
(ii) It does not react with any physical state of water.
(b) When calcium metal is added to water the gas evolved does not catch fire but the same gas evolved on adding sodium metal to water catches fire. Why is it so?
Answer: (a) (i) Iron metal does not react with cold aswell as hot water but it reacts with steam.
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)↑
(ii) Gold does not react with cold water, hot water and not even with steam.
(b) When calcium metal is added to water the hydrogen gas evolved, does not catch fire because the reaction of calcium with water is less violent and the heat evolved is not sufficient for the hydrogen gas to catch fire. But the same gas evolved on adding sodium metal to water, catches fire because the reaction is very violent and highly exothermic and the heat evolved is sufficient for hydrogen gas to catch fire.
Question. Give reason for the following observation :
Copper vessels get a green coat when left exposed to air in rainy season.
Answer: Copper vessels get a green coat when left exposed to air in rainy season due to the formation of CuCO3 .Cu(OH)2.

Question. (a) What are amphoteric oxides? Choose the amphoteric oxides from amongst the following oxides :
Na2O, ZnO, Al2O3, CO2, H2O
(b) Why is it that non-metals do not displace hydrogen from dilute acids?
Answer: (a) Those oxides which react with both acids as well as bases to produce salts and water are called amphoteric oxides.
Among the given oxides, Al2O3 and ZnO are amphoteric in nature.
(b) Non-metals do not displace hydrogen from dilute acids because non-metals do not provide electrons to change H+ ions into hydrogen gas.
Question. Explain how the following metals are obtained from their compounds by the reduction process :
(i) Metal M which is in the middle of the reactivity series.
(ii) Metal N which is high up in the reactivity series.
Give one example of each type.
Answer: (i) Those metals (M) which have moderate reactivity such as zinc, lead, iron, copper, etc.
are obtained by the reduction of their oxides by reducing agents such as carbon or aluminium. e.g.,
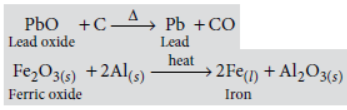
(ii) Those metals (N) which are high up in the reactivity series are extracted by electrolytic reduction method.
e.g., sodium is obtained by the electrolysis of its molten chloride. the sodium metal is deposited at the cathode, whereas chlorine is liberated at the anode.
At cathode : Na+ + e– Na
At anode : 2Cl– Cl2 + 2e–
Question. What changes in the colour of iron nails and copper sulphate solution do you observe after keeping the iron nails dipped in copper sulphate solution for about 30 minutes?
Answer: As iron (Fe) is more reactive than copper (Cu) so, iron displaces copper from its salt solution.
Thus, blue coloured CuSO4 gets converted into pale green coloured FeSO4 after keeping iron nails in CuSO4 solution.
CuSO4+Fe→ FeSO4 + Cu
Blue Pale green
Question. Which gas is generally liberated when a dilute solution of hydrochloric acid reacts with an active metal?
Answer: Generally, hydrogen gas is liberated when dilute solution of hydrochloric acid reacts with active metals.
Question. Arrange the following metals in a decreasing order of activity :
Na, K, Cu, Ag
Answer: The decreasing order of activity is K > Na > Cu > Ag
Question. (a) Show the formation of Na2O by the transfer of electrons between the combining atoms.
(b) Why are ionic compounds usually hard?
(c) How is it that ionic compounds in the solid state do not conduct electricity and they do so when in molten state?
Answer: (a) The formation of Na2O can be represented as :
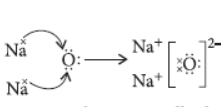
(b) Ionic compounds are usually hard due to strong forces of attraction between oppositely charged ions.
(c) Ionic compounds in the solid state do not conduct electricity because movement of ions in solid state is not possible due to the rigid structure.
But they conduct electricity in the molten state as the electrostatic forces of attraction between oppositely charged ions are overcome by heat and ions become free to move.
Question. Write the names and symbols of two most reactive metals belonging to group I of the periodic table. Explain by drawing electronic structure how either one of the two metals reacts with a halogen. With which name is the bond formed between these elements known and what is the class of the compound so formed known? State any four physical properties of such compounds.
Answer: Rubidium (Rb) and Caesium (Cs) are the two most reactive metals belonging to group-I of the periodic table.
Caesium (Cs) reacts with halogen say chlorine (Cl) to form caesium chloride as follows :
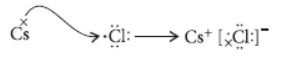
This bond formed by complete transfer of electrons between two elements is called ionic bond and the compound so formed is known as ionic compound.
Physical properties of ionic compounds are :
(i) Physical nature : Ionic compounds are solids and are somewhat hard because of the strong forces of attraction between the positive and negative ions.
(ii) Melting and boiling points : Ionic compounds have high melting and boiling points.
This is because a considerable amount of energy is required to break the strong inter-ionic forces of attraction.
(iii) Solubility : They are soluble in water and insoluble in solvents such as kerosene, petrol, etc.
(iv) Conduction of electricity : A solution of an ionic compound in water contains ions which move to the opposite electrodes when electricity is passed through the solution. They conduct electricity in molten state as well as in aqueous solution but not in solid state because movement of ions in the solid state is not possible due to their rigid structures.
Question. Name a reducing agent that may be used to obtain manganese from manganese dioxide.
Answer: Aluminium reduces manganese dioxide (MnO2) to manganese (Mn). The reaction is highly exothermic.
3MnO2(s) + 4Al(s) heat→ 3Mn(l) + 2Al2O3(s)
Question. From amongst the metals sodium, calcium, aluminium, copper and magnesium, name the metal
(i) which reacts with water only on boiling and
(ii) another which does not react even with steam.
Answer: (i) Aluminium is the metal which reacts with water only on boiling (i.e., it reacts with steam).
(ii) Copper metal does not react even with steam due to its low reactivity.
Question. Give reason for the following observation :
ionic compounds in general have high melting and boiling points.
Answer: Due to strong forces of attraction, the ions are bound to each other very firmly. As a result, the electrovalent or ionic solids have high melting and boiling points.
Question. Give reason for the following observation :
highly reactive metals cannot be obtained from their oxides by heating them with carbon.
Answer: The oxides of the most reactive metals such as sodium, potassium, magnesium, aluminium, etc. cannot be reduced by reducing agents such as carbon or aluminium. This is because these metals have more afinity for oxygen than carbon or aluminium hence, cannot be reduced by common reducing agents.
Question. Name two metals which react violently with cold water. Write any three observations you would make when such a metal is dropped into water. How would you identify the gas evolved, if any, during the reaction?
Answer: Sodium and potassium react with cold water violently.
When these metals are dropped into water then following changes are observed :
(i) Bubbles come out of water due to the evolution of a gas.
(ii) This gas catches fire immediately.
(iii) The beaker becomes hot as the reaction is highly exothermic.
When a burning matchstick is brought near this gas (hydrogen) then it burns explosively with a ‘pop’ sound.
Question. What is meant by refining of metals? Name the most widely used method of refening impure metals produced by various reduction processes. Describe with the help of a labelled diagram how this method may be used for refining of copper.
Answer: The process of purifying the impure (crude) metal is called refining of metals.
The most widely used method of refining impure metals produced by various reduction processes is electrolytic refining.
In electrolytic refining, a thick block of impure metal acts as anode. It is connected to the positive terminal of the battery. A thin sheet of pure metal acts as cathode. It is connected to the negative terminal of the battery. An aqueous solution of a suitable salt of the metal is used as the electrolyte.
On passing current through the electrolyte, pure metal gets deposited on the cathode and the impure metal of the anode dissolves into the elctrolyte. The impurities either dissolve in the solution or settle down at the bottom of the anode as anode mud.

Question. (a) Distinguish between ‘roasting’ and ‘calcination’. Which of these two is used for sulphide ores and why?
(b) Write a chemical equation to illustrate the use of aluminium for joining cracked railway lines.
(c) Name the anode, the cathode and the electrolyte used in the electrolytic refining of impure copper.
Answer: (a) The process of heating an ore (generally a sulphide ore) strongly below its melting point in the presence of an excess of air is called roasting.
Calcination is the process of heating an ore (generally a carbonate ore) strongly in the absence of air or very limited supply of air.
Roasting process is used for sulphide ores because sulphur gets oxidised to SO2 which can be easily removed leaving behind the metal oxide.
(b) Aluminium displaces iron from iron oxide on heating.
Fe2O3(s) + 2Al(s) heat→ Al2O3(s) + 2Fe(l) + Heat
This reaction produces lots of heat which results in the melting of railway track lines. After cooling, iron again forms a hard solid and hence, cracked railway lines can be joined.
(c) For electrolytic refining of impure copper, impure copper is used as anode, pure copper is used as cathode and copper sulphate solution is used as the electrolyte.
Question. What is meant by ‘rusting’? With labelled diagrams describe an activity to find out the conditions under which iron rusts.
Answer: Iron when exposed to moist air for a long time acquires a coating of a brown flaky substance known as rust and this process is called rusting.
Following activity can be performed to find out the conditions under which iron rusts :
Materials required : Iron nails, distilled water, turpentine oil, anhydrous calcium chloride.
Procedure :
1. Take three test tubes and put one clean nail in each of them. Label them as A, B and C.
2. Pour some water in test tube A. In test tube B, pour some boiled distilled water along with some turpentine oil. In test tube C, add some anhydrous calcium chloride.
3. Leave these test tubes undisturbed for a few days.
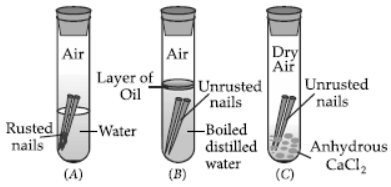
Observations : Only in test tube A, iron nails get rusted since the nails in this test tube are exposed to both air and water.
Conclusion : Both air and water are required for rusting of iron.
Question. No chemical reaction takes place when granules of a solid, A, are mixed with the powder of another solid, B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in the molten state while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(i) Based on the given information make an assumption about A and B and write a chemical equation for the chemical reaction indicating the conditions of reaction, physical state of reactants and products and thermal status of reaction.
(ii) Mention any two types of reactions under which above chemical reaction can be classified.
Answer: From the given information it is clear that A is Fe2O3 and B is Al.
(i) When A (Fe2O3) and B (Al) are heated then C (Fe) metal is formed which settles down in the molten state while D (Al2O3) floats over it. This reaction is exothermic in nature.
Fe2O3(s) + 2Al(s) heat→ 2Fe(l) + Al2O3(s)
(ii) This reaction can be classified under displacement reaction and redox reaction.
Question. Give reason why
(i) metals are good conductors, whereas non-metals are bad conductors of electricity.
(ii) metals replace hydrogen from acids whereas non-metals do not.
(iii) an iron knife dipped in a blue copper sulphate solution turns the blue solution light green.
(iv) sodium is kept under kerosene.
(v) carbon cannot reduce the oxides of sodium or aluminium.
Answer: (i) Metals are good conductors of electricity because they have free electrons or ions while non-metals do not contain free electrons or ions.
(ii) When a metal (which is more electropositive than hydrogen) is placed in an acid solution, it loses electrons. These electrons are gained by H+ ions to produce hydrogen gas. e.g.,

Non-metals do not have tendency to lose electrons.
(iii) As iron is more reactive than copper so, iron will displace copper from copper sulphate solution.
Fe CuSO4 → FeSO4 + Cu
Blue Pale green
Thus, blue coloured copper sulphate gets converted into light green iron sulphate.
(iv) Sodium catches fire vigorously on reaction with oxygen at room temperature if kept in open.
Therefore, sodium is kept under kerosene.
(v) Carbon cannot reduce the oxides of sodium or aluminium because sodium and aluminium are placed at the top of the reactivity series and are highly reactive. They have more affinity for oxygen than carbon.



