Please refer to Acids Bases Salts Class 10 Science notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Science books for Class 10. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 10 Science Acids Bases Salts Notes and Questions
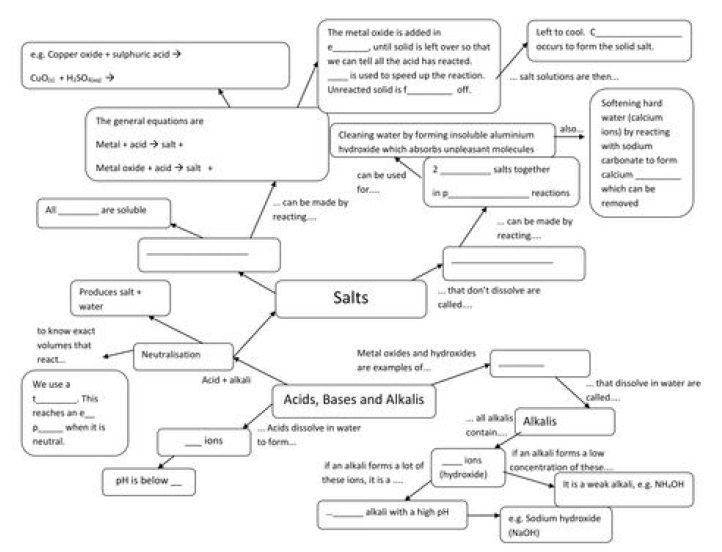
Acids: Substances which turn blue litmus solution red are called acids. Acids are sour in taste Bases: Substances which change red litmus solution blue are called bases. They are bitter in taste.
Mineral Acids: Acids which are obtained from minerals like sulphates, nitrates, chlorides etc. are called mineral acids, e.g., H2SO4(Sulphuric acid), HNO3(Nitric acid) and HCl (Hydrochloric acid).
Organic Acids: Acids which are obtained from plants and animals are called organic acids.e.g. citric acid, ascorbic acid, tartaric acid, lactic acid, acetic acid. Hydronium Ions (H3O + ): They are formed by reaction of H+ (from acid) and H2O. It is because H+ is unstable.
Strong Acids: Acids which dissociate into ions completely are called strong acids. E.g. H2SO4, HCl
Weak Acids: Acids which do not dissociate into ions completely are called weak acids E.g. Citric acid, acetic acid.
Strong bases: The bases in which complete dissociation of hydroxide ions takes place are called Strong Bases. E.g. NaOH, KOH
Weak bases: The bases which do not dissociate completely in aqueous solution to form hydroxide ions are called weak Bases. E.g. NH4OH
Chemical properties of acids
(i) Acids react with active metals to give salt and hydrogen gas.
(ii) Acids react with metal carbonate and metals hydrogen carbonate to give salt, water and carbon dioxide.
(iii) Acids react with bases to give salt and water. This reaction is called neutralization reaction.
iv) Acids react with metals oxides to give salt and water.
Chemical properties of Bases
(i) Reaction with Metals – Certain metals such as Zinc, Aluminum and Tin react with alkali solutions on heating and hydrogen gas is evolved
(ii) Reaction with acids – Bases react with acids to form salt and water
Indicators – Indicators are substances which indicate the acidic or basic nature of the solution by their color change.
Olfactory Indicator: Substances which change their smell when mixed with acid or base are known as Olfactory Indicators. For example; Onion, vanilla etc.
Onion: Paste or juice of onion loses its smell when added with base. It does not change its smell with acid.
Vanilla: The smell of vanilla vanishes with base, but its smell does not vanish with an acid.
Olfactory Indicators are used to ensure the participation of visually impaired students in the laboratory
Universal Indicator: A universal indicator is a mixture of indicators which shows a gradual but wellmarked series of color changes over a very wide range of change in concentration of H+ ion.
pH scale: A scale for measuring hydrogen ion concentration in a solution. The pH of a solution is defined as the negative logarithm of hydrogen ion concentration in moles per litre.

PH =-log [H+] pH =-log [H3O +] where [H+] or [H3O +] represents concentrations of hydrogen ions in solution. The pH of a neutral solution is 7 the pH of an acidic solution is < 7 The pH of a basic solution is> 7
Neutral, Acidic and Basic Salts:
(i) Neutral Salt: Salts produced because of reaction between a strong acid and strong base are neutral in nature. The pH value of such salts is equal to 7, i.e. neutral.
Example: Sodium chloride, Sodium sulphate. Potassium chloride, etc.
(ii)Acidic Salts: Salts which are formed after the reaction between a strong acid and weak base are called Acidic salts. The pH value of acidic salt is lower than 7. For example, Ammonium sulphate, Ammonium chloride, etc.
iii) Basic Salts: Salts which are formed after the reaction between a weak acid and strong base are called Basic Salts. For example; Sodium carbonate Sodium acetate, etc.





The water of Crystallization: Many salts contain water molecule and are known as Hydrated Salts. The water molecule present in salt is known as Water of crystallization.
Examples:
Copper sulphate pentahydrate (CuSO4.5H2O): Blue color of copper sulphate is due to presence of 5 molecules of water. When copper sulphate is heated, it loses water molecules and turns: into grey – white colour, which is known as anhydrous copper sulphate. After adding water, anhydrous copper sulphate becomes blue again.
EQUATIONS OF ACIDS, BASES AND SALTS
1. Acid + Metal → Salt + Hydrogen gas- H2SO4+Zn → ZnSO4+H2
2. Base+ Metal → Salt + Hydrogen gas – Na2ZnO2 + H2 → 2NaOH + Zn
3. Salt + Water → Base + Acid – NaCl (aq) + H2O (l) → NaOH (aq) +HCl (aq)
4. Acids give hydronium ions in water- HCl+H2O —>H3O+ + Cl
5. Bases generate OH- ions in water NaOH(s)+H2O → Na+ (aq)+OH– (aq)
Short Answer Type Questions
Question. Write the name of the products formed by heating gypsum at 373K.Write one use of it.
Ans: Plaster of Paris and water. It is used for plastering fractured bone.
Question. Write the chemical name and formula of the compound which is used as an antacid.
Ans: Sodium bicarbonate, NaHCO3
Question. Given below are the pH values of different liquids.7.0, 14.0, 4.0, and 2.0. Which of these could be that of-
a) lemon juice
b) distilled water
c) sodium hydroxide solution
d) tomato juice.
Ans: a) lemon juice- 2.0
b) distilled water-7.0
c) sodium hydroxide solution `14.0
d) tomato juice- 4.0 18
Question. What is baking powder? How does it make the cake soft and spongy?
Ans: Baking powder is a mixture of sodium hydrogen carbonate and tartaric acid. On heating it liberates CO2 which makes the cake soft and spongy
Question. Write the chemical name of Plaster of Paris. Write a chemical equation to show the reaction between Plaster of Paris and water. Name the compound produced in this reaction.
Ans: Calcium Sulphate hemihydrates.
CaSO4. 1 /2H2O +1 /2H2O–→ CaSO4.2H2O
The compound produced is Gypsum.
Question. A gas X reacts with lime water and forms a compound Y which is used as bleaching agent in the chemical industry. Identify X and Y. Give the chemical equation of the reaction involved.
Ans: X is chlorine Y is CaOCl2 (calcium oxy chloride) used as bleaching agent.
Ca (OH)2+ Cl2—–→ CaOCl2 +H2O
Long Answer Type Questions
Question. a) A milk man adds a very small amount of baking soda to fresh milk. Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
b) Mention pH range within which our bodyworks?
c) Explain how antacids give relief from acidity.
d) Mention the nature of tooth pastes. How do they prevent tooth decay?
Ans: a) It is done to prevent the formation of lactic acid which spoils the milk
b) pH range 7.0- 7.8
c) Antacids neutralizes excess of acid in our body and gives relief.
d) Basic. Neutralize the acid formed in the mouth
Question. a) Crystals of a substance changed their color on heating in a closed test tube but regained it after some time when they were allowed to cool down. Name the substance and write its formula. Explain the phenomenon.
b) How is sodium carbonate prepared? Give two uses of the compound
Ans: a) Copper sulphate, CuSO4.5H2O. It is blue. It becomes white on heating due to loss of water molecule. CuSO4.5H2O —Heat–> CuSO4+5 H2O It regains its colour by absorbing water from atmosphere CuSO4+ 5 H2O –→ CuSO4.5H2O
b) Sodium carbonate is prepared by passing CO2 through ammoniac brine. Used for production of washing powder & manufacture of glass
Multiple Choice Questions
Question. Which of the following gives the correct increasing order of acidic strength?
a) Water< Acetic acid < Water < Hydrochloric acid
b) Hydrochloric acid < water < Acetic acid 19
Answer
A
Question. Which of the following salts does not contain water of crystallization?
a) Blue vitriol
b) Baking soda
c) Gypsum
d) Washing soda
Answer
B
Question. Common salt, besides used in kitchen, can also be used as the raw material for making:
i) Washing soda ii) bleaching powder iii) Baking soda iv) slaked lime
a) i) and ii)
b) i) and iii)
c) i), ii) and iii)
d) i),iii) and iv)
Answer
C
Question. The acid having highest hydrogen ion concentration is one with
a) pH=2.5
b) pH = 1.8
c) pH=7
d) pH=10
Answer
B
Question. The pH of gastric juices released during digestion is:
a) less than 7
b) more than 7
c) Equal to 7
d) equal to 0
Answer
A
Reasoning and assertion type questions
The following questions consists of two statements- Assertion (A) and Reason(R). Answer these questions selecting appropriate option given below:
a) Both A and R are true and R is correct explanation of A
b) Both A and R are true and R is not correct explanation of A
c) A is true but R is false
d) A is false but R is true
Question. Assertion (A) – The aqueous solutions of glucose and alcohol do not show acidic character.
Reason (R) – Aqueous solutions of glucose and alcohol do not give H+ ions.
Ans: a) Both A and R are true and R is correct explanation of A
Question. Assertion (A) – Carbonic acid is weak acid.
Reason (R) – It ionized completely in aqueous solution.
Ans: c) A is true but R is false
CCT Type Questions
Question. Ritesh was asked to determine the melting point of a given organic solid. For this, he used a bath containing cone. H2SO4. When he was looking at the thermometer, he lost his concentration and became a little casual. The beaker containing boiling Sulphuric acid fell on his clothes. His clothes were burnt and he got severe burns on hands.
1. Why did Ritesh meet with an accident?
2. Why did he get severe burns on the hands?
3. What lessons can a student learn from the above episode?
Ans: 1. The bath or the beaker containing cone. H2SO4 was not properly placed on the tripod stand. It lost balance and the acid fell over him.
2. Sulphuric acid is a very powerful dehydrating agent. It removed water contents from the skin which got charred and burns appeared on hands.
3. While working in the chemistry laboratory, a student must observe the following precautions:
1. Must always wear an appron in the laboratory.
2. Must always keep some distance from the table where the experiment is performed.
3. Must always remain alert in the laboratory and must not lose concentration.
Question. Ram was suffering from a stomach pain for a number of days. He consulted a doctor who advised him to take two antacid tablets after each meal for about a week and avoid spicy food. Ram followed the advice strictly and was cured.
1. What was the problem faced by Ram?
2. How did doctor help him?
3. Write the chemical equation if any
4. What is the value associated with this?
Ans: 1. Ram had developed acidity in the stomach resulting in the formation of small ulcers which caused pain.
2. The antacid tablets contain base like NaHCO3 or Mg (OH)2 which neutralize the effect of HCl released in the stomach.
3. NaHCO3 + HCl ———–> NaCl + H2O + CO2 (Antacid)
4. As far as possible, one should always avoid spicy and fried food stuff. They create acidity in the stomach. This leads in the formation of small ulcers which give pain. Apart from that one must always keep some antacid tablets or liquid gels.
Question. Kamla was playing in the garden. She was stung by a wasp and started crying. Her mother immediately applied a coating of tooth paste on the affected area and then took her to the doctor.
1. Why did Kamla cry?
2. What does wasp sting contain?
3. Why did her mother apply tooth paste on the affected area?
4. What values are displayed by this episode?
Ans:
1. Kamla cried because the sting by the wasp is very painful.
2. Wasp sting contains in its formic acid (HCOOH)
3. Tooth paste contains in it some basic ingredients which neutralize the effect of formic acid (HCOOH) and give relief.
4. Kamla’s mother had knowledge of chemistry. Tooth paste is readily available and she gave a first aid to her daughter.

We hope the above Acids Bases Salts Class 10 Science are useful for you. If you have any questions then post them in the comments section below. Our teachers will provide you an answer. Also refer to MCQ Questions for Class 10 Science


