Please refer to Chemical Reactions and Equations Class 10 Science notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Science books for Class 10. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 10 Science Chemical Reactions and Equations Notes and Questions
Chemical Reaction: In a chemical reaction, a new substance is formed which is completely different in properties from the original substance, so in a chemical reaction, a chemical change takes place.
Example: The burning of magnesium in the air to form magnesium oxide is an example of a chemical reaction.
2Mg(s) + O2(g) △→ 2MgO(s)
Before burning in air, the magnesium ribbon is cleaned by rubbing with sandpaper.
This is done to remove the protective layer of basic magnesium carbonate from the surface of the magnesium ribbon.
Characteristics of Chemical Reactions:
(i) Evolution of gas
(ii) Change in Colour
(iii) Change in state of substance
(iv) Change in temperature
(v) Formation of precipitate
Balanced Chemical Equation: A balanced chemical equation has the number of atoms of each element equal on both sides.
Example: Zn + H2SO4 → ZnSO4 + H2
Types of Chemical Reactions:
Chemical reactions can be classified in following types:
(i) Combination Reaction: Reactions in which two or more reactants combine to form one product are called Combination Reactions.
Example:
When magnesium is burnt in the air (oxygen), magnesium oxide is formed. In this reaction, magnesium is combined with oxygen.
Mg(s) + O2(g) → 2MgO(s)
Magnesium + Oxygen → Magnesium Oxide
(ii) Decomposition Reaction: Reactions in which one compound decomposes in two or more compounds or elements are known as Decomposition Reaction. A decomposition reaction is just the opposite of combination reaction.
Example:
When calcium carbonate is heated, it decomposes into calcium oxide and carbon dioxide.
CaCO3(s) heat−→− CaO(s) + CO2(g)
Calcium carbonate → Calcium oxide + Carbon dioxide
Thermal Decomposition: The decomposition of a substance on heating is known as Thermal Decomposition.
Example: 2Pb (NO3)2(s) heat−→− 2PbO(s) + 4NO2 (g) + O2(g)
Electrolytic Decomposition: Reactions in which compounds decompose into simpler compounds because of passing of electricity, are known as Electrolytic Decomposition. This is also known as Electrolysis.
Example: When electricity is passed in water, it decomposes into hydrogen and oxygen.
2H2O (l) → 2H2 (g) + O2(g)
Photolysis or Photo Decomposition Reaction: Reactions in which a compound decomposes because of sunlight are known as Photolysis or Photo Decomposition Reaction.
Example: When silver chloride is put in sunlight, it decomposes into silver metal and chlorine gas.
2AgCl(s) (white) Sunlight−→−−−−− 2Ag(s) (grey) + Cl2 (g)
(iii) Displacement Reaction: The chemical reaction in which a more reactive element displaces a less reactive element from a compound is known as Displacement Reactions. Displacement reactions are also known as Substitution Reaction or Single Displacement/ replacement reactions.
Example:
When zinc reacts with hydrochloric acid, it gives hydrogen gas and zinc chloride.
Zn(s) + 2HCl (aq) → ZnCl2 (aq) + H2(g)
(iv) Double Displacement Reaction: Reactions in which ions are exchanged between two reactants forming new compounds are called Double Displacement Reactions.
Example:
When the solution of barium chloride reacts with the solution of sodium sulphate, white precipitate of barium sulphate is formed along with sodium chloride.
BaCl2 (aq) + Na2SO4 (aq) → BaSO4(s) (Precipitate) + 2NaCl (aq)
Precipitation Reaction: The reaction in which precipitate is formed by the mixing of the aqueous solution of two salts is called Precipitation Reaction.
Example:

Neutralization Reaction: The reaction in which an acid reacts with a base to form salt and water by an exchange of ions is called Neutralization Reaction.
Example:
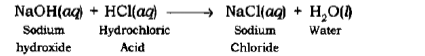
(v) Oxidation and Reduction Reactions:
Oxidation: Addition of oxygen or non-metallic element or removal of hydrogen or metallic element from a compound is known as Oxidation.
Elements or compounds in which oxygen or non-metallic element is added or hydrogen or metallic element is removed are called to be oxidized.
Reduction: Addition of hydrogen or metallic element or removal of oxygen or non-metallic element from a compound is called Reduction.
The compound or element which goes under reduction in called to be reduced.
Oxidation and Reduction take place together.
Oxidizing agent:
* The substance which gives oxygen for oxidation is called an Oxidizing agent.
* The substance which removes hydrogen is also called an Oxidizing agent.
Reducing agent:
* The substance which gives hydrogen for reduction is called a Reducing agent.
* The substance which removes oxygen is also called a Reducing agent.
REDOX REACTIONS: The reaction in which oxidation and reduction both take place simultaneously is called Redox reaction.
When copper oxide is heated with hydrogen, then copper metal and hydrogen are formed.
CuO + H2 → Cu + H2O
(i) In this reaction, CuO is changing into Cu. Oxygen is being removed from copper oxide. Removal of oxygen from a substance is called Reduction, so copper oxide is being reduced to copper.
ii) In this reaction, H2 is changing to H2O. Oxygen is being added to hydrogen. Addition of oxygen to a substance is called Oxidation, so hydrogen is being oxidized to water.
* The substance which gets oxidized is the reducing agent.
* The substance which gets reduced is the oxidizing agent
(vi) Exothermic and Endothermic Reactions:
Exothermic Reaction: Reaction which produces energy is called Exothermic Reaction. Most of the decomposition reactions are exothermic.
Example:
Respiration is a decomposition reaction in which energy is released.

Endothermic Reaction: A chemical reaction in which heat energy is absorbed is called Endothermic Reaction.
Example: Decomposition of calcium carbonate.

Effects of Oxidation Reactions in Everyday life: Corrosion and Rancidity.
Corrosion: The process of slow conversion of metals into their undesirable compounds due to their reaction with oxygen, water, acids, gases etc. present in the atmosphere is called Corrosion.
Rusting: Iron when reacts with oxygen and moisture forms red substance which is called Rusting.

Example: Rusting of iron.
Corrosion of Copper: Copper objects lose their lustre and shine after some time because the surface of these objects acquires a green coating of basic copper carbonate,
CuCO3.Cu (OH)2 when exposed to air.
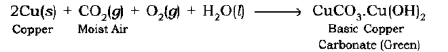
Corrosion of Silver Metal: The surface of silver metal gets tarnished (becomes dull) on exposure to air, due to the formation of a coating of black silver sulphide(Ag2S) on its surface by the action of H2S gas present in the air.
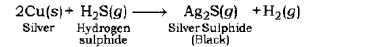
Rancidity: The taste and odour of food materials containing fat and oil changes when they are left exposed to air for a long time. This is called Rancidity. It is caused due to the oxidation of fat and oil present in food materials.
Methods to prevent rancidity:
* By adding anti-oxidant.
* Vacuum packing.
* Replacing air by nitrogen.
Refrigeration of foodstuff.

Short Answer Type Questions
Question. List four observations that help us to determine whether a chemical reaction has taken place.
Answer. (i) Evolution of gas
(ii) Change in temperature
(iii) Change in state
(iv) Change in color
Question. Why do we store silver chloride in dark colored bottles? Explain in brief.
Answer. Silver chloride on exposure to sunlight may decompose as per the following reaction
2AgCL → 2Ag + Cl2
Therefore it is stored in dark colored bottles.
Question. Define a combination reaction. Give one example of a combination reaction which is also exothermic.
Answer. A combination reaction is said to have occurred when two or more than two substances combine to form a single substance.
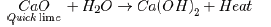
Question. What happens when an iron nail is put inside the copper sulfate solution? Write a reaction with observation.
Answer. Iron nail turns brown, blue color of CuSO4 changes to colorless. (or light green)
Fe(s)+CuSO4(aq)→FeSO4(aq)-+Cu(s)
Question. When Hydrogen gas is passed over heated copper (II) oxide, copper and steam are formed. Write the balanced chemical equation with physical states for this reaction. State what kind of chemical reaction is this?
Answer. CuO(s) + H2(g) —Heat→ Cu(s) + H2O(g)
This is a Redox reaction.
Question. Why eatables are preferably packed in aluminum foils?
Answer: Aluminum foils do not corrode in atmosphere even if kept for a long time. Actually, a protective coating of aluminum oxide (Al2O3) is formed on the surface of the metal. It stops any further reaction of the metal with air (oxygen) and water. The eatables do not get spoiled.
Question. Why decomposition reactions are called the opposite of combination reactions? Write equations for these reactions.
Answer: A decomposition reaction may be defined as the reaction in which a single substance decomposes or splits into two or more substances under suitable conditions.
For example,

Question. Identify the substance oxidised and substance reduced in the following reactions
(i) ZnO(s) + C(s) ———> Zn(s) + CO(g)
(ii) 2Na(s) + O2(g) ———> 2Na2O(s)
(iii) CuO(s) + H 2(g) ———> Cu(s) + H2O(l).
Answer:
(i) C is oxidized to CO and ZnO is reduced to Zn.
(ii) Na is oxidized to Na2O and O2 is reduced.
(iii) H2 is oxidized to H2O and CuO is reduced to Cu.
Question.
(a) Why is combustion reaction an oxidation reaction?
(b) How will you test whether the gas evolved in a reaction is hydrogen?
(c) Why does not silver evolve hydrogen on reacting with dilute sulphuric acid?
Answer:
(a) Combustion reaction is an oxidation reaction because it is always carried in the presence of air or oxygen. For example,
CH4(s) + 2O2(g) ——–> CO2(g) + 2H2O (l)
(b) Bring a burning match stick close to the mouth of the tube from which hydrogen gas escapes. The gas will immediately catch fire and this will be accompanied by pop sound.
(c) Silver is a less reactive metal in the sense that it occupies a place below hydrogen in the reactivity series. Therefore, it does not evolve hydrogen gas on reacting with either dilute sulphuric acid or dilute hydrochloric acid.
Question. Identify the type of reaction in the following examples :
(i) Na2SO4(aq) + BaCl2(aq) ———-> BaSO4(s) + 2NaCl(aq)
(ii) Fe(s) + CuSO4(aq) ———-> FeSO4(aq) + Cu(s)
(iii) 2H2(g) + O2(g) ———> 2H2O(l)
Answer:
(i) It is an example of double displacement reaction.
(ii) It is an example of displacement reaction.
(iii) It is an example of combination reaction.
Long Answer Type Questions
Question.(a) Why cannot a chemical change be normally reversed?
(b) Why is it always essential to balance a chemical equation?
(c) Why do diamond and graphite, the two allotropic forms of carbon evolve different amounts of heats on combustion?
(d) Can rusting of iron take place in distilled water?
Answer: (a) In a chemical change, the products are quite different from the reactants. Therefore, it cannot be normally reversed.
(b) A chemical equation has to be balanced to meet the requirement of the law of conservation of mass. According to the law, the total mass of the reacting species taking part in the reaction is the same as that of the products formed. Since there is a direct relationship between the mass of the different species and their number, it is always essential to balance a chemical equation.
(c) Because they differ in the arrangement of carbon atoms present and have different shapes. The attractive forces among the atoms in the two cases are not same. That is why they evolve different amount of heat.
C(diamond) + O2(g) ———–> CO2(g) + 393.5 kj
C(graphite) + O2(g) ———–> CO2(g) + 395.4 kj
Please note that diamond and graphite are the two allotropic forms of carbon.
(d) No, rusting of iron cannot take place in distilled water because it neither contains dissolved oxygen nor carbon dioxide. Both are essential for the rusting of iron.
Question. You are given the following materials:
(i) Iron nails
(ii) Copper sulphate solution
(iii) Barium chloride solution
(iv) Copper powder
(v) Ferrous sulphate crystals
(vi) Quick lime.
Identify the type of chemical reaction taking place when :
(a) Barium chloride solution is mixed with copper sulphate solution and a white precipitate is observed.
(b) On heating, copper powder in air in a china dish, the surface of copper powder becomes black.
(c) On heating green ferrous sulphate crystals, reddish brown solid is left and a gas having smell of burning sulphur is noticed.
(d) Iron nails when left dipped in blue copper sulphate solution become brownish in colour and blue colour of copper sulphate solution fades away.
(e) Quick lime reacts vigorously with water releasing a large amount of heat.
Answer:

Question. A silvery white metal X is in the form of ribbons. Upon ignition, it burns with a dazzling white flame to form white powder Y. When water is added to the powder Y, it partially dissolves to form a substance Z which is used as an antacid.
(a) What is metal X?
(b) Name the white powder Y.
(c) What is the substance Z ?
(d) Write the chemical reactions that are taking place.
Answer:
(a) The metal is X is Mg.
(b) The white powder Y is MgO.
(c) White powder Y dissolves partially in water to form substance Z. It is Mg (OH), and is used as an antacid.
(d) The chemical reactions that are taking place are :
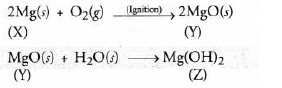
Question. (i) Account for the following :
(a) White silver chloride turns grey in sunlight.
(b) Brown coloured copper powder on heating in air turns into black coloured substance.
(ii) What do you mean by
(a) Displacement reaction
(b) Reduction reaction
(c) Combination reaction?
Write balanced chemical equation in support for all
Answer:
(i) (a) White coloured silver chloride undergoes decomposition in the presence of sunlight and forms silver (grey in colour) and chlorine.

(b) Brown coloured copper powder on heating in air gets oxidised to copper oxide which is black in colour.
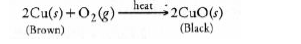
(ii) For the different types of reactions,
(a) In a displacement reaction, one element takes the place of another in a compound dissolved in a solution. For example,
Fe(s) + CuSO4 (aq) ———> FeSO4 (aq) + Cu(s)
(b) Combination reaction may be defined as the reaction in which two or more substances combine under suitable conditions to form a new substance. For example,

(c) A decomposition reaction may be defined as the reaction in which a single substance decomposes or splits into two or more substances under suitable conditions.
For example,
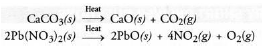
It may be concluded that a certain substance is formed or synthesised in combination reaction and it breaks or splits in decomposition reaction. Therefore, the two reactions oppose each other.
Question. Observe the given figure and answer the following questions.
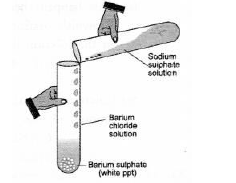
(a) Write the complete balanced reaction for the reaction that takes place.
(b) Type of reaction involved.
(c) Is there any precipitate formed?
(d) If any precipitate formed, write the color of the precipitate.
Answer:

(b) It is a double displacement reaction
(c) Yes, a precipitate of barium sulphate is formed.
(d) The precipitate is white in colour.
Assertion-Reason Type Questions:
Reasoning and assertion type questions: The following questions consists of two statements-
Assertion (A) and Reason(R). Answer these questions selecting appropriate option given below:
a) Both A and R are true and R is correct explanation of A
b) Both A and R are true and R is not correct explanation of A
c) A is true but R is false
d) A is false but R is true
1. Assertion (A) – Calcium Carbonate when heated gives calcium oxide and water
Reason (R) – on heating CaCO3, decomposition reaction takes place.
Answer: 1. d) A is false but R is true
2. Assertion (A) – White silver chloride turns grey in sunlight.
Reason (R) – Decomposition of silver chloride in presence of sunlight takes place to form silver metal and chlorine gas.
Answer. a) Both A and R are true and R is correct explanation of A
CCT Based Questions:
Question. A lady wanted to give a coating of white wash to her room. She purchased quick lime from the market and dissolved it in water and immediately applied the same on the wall. In this process, she spoiled her hands and even suffered minor burns. Her friend advised her not be in haste and keep the container overnight before applying a coating on the wall. She followed her advice and there was now no problem.
1. What mistake was committed by the lady?
2. Why did she suffer from burns?
3. Why was so much heat evolved?
4. Write the balanced equation for the reaction involved
Answer: The lady should have waited for a few hours because when quick lime is dissolved in water, slaked lime is formed and this process is highly exothermic.
2. The solution might have become very hot and that is why the lady suffered from burns.
3. Quick lime is CaO and it reacts with water to form Ca(OH)2 which is known as slaked lime. The dissolution process is highly exothermic. That is why so much heat was evolved. By keeping container overnight, the chemical reaction subsided and now there was no problem to apply the coating of white wash on the wall. In this way, she rendered service to the lady
CaO(s) + H2O(l) → Ca(OH)2(s) + heat
4. Quick lime
Question. Mohan was working in a factory. He purchased a new cycle but kept it in the open. After two months he found that the cycle chain and even the handles got rusted. His friend advised him to apply a coating of rust proof paint to the cycle and not to keep it in the open in future.
1. Why was the cycle rusted?
2. What is the role of rust proof paint?
3. What values are associated with this gesture?
Answer:
1. Air contains both oxygen and moisture. In their presence iron slowly got rusted

2. The coating of rust proof paint checked further corrosion. Similarly, by keeping the cycle under a covered shed, rusting can be avoided.
3. He played the role of a sincere friend and gave a very sincere advice to Mohan.
Question. A student working in the laboratory prepared an aqueous solution of silver nitrate and kept it in a glass beaker overnight. Next morning, he found that the beaker has developed black turbidity.
1. Why did the solution develop black turbidity?
2. In your opinion, what precaution he should have taken?
Answer:
1. The silver salts are sensitive to light. When kept exposed to light for a few hours silver nitrate decomposes and a black turbidity appears.
2. The student should have covered the beaker from outside with the help of a brown paper to avoid direct action of sun light.
Question. Sonia purchased a packet of potato chips from the shop. She opened the packet and ate some of the chips and left the packet as such in one comer of her study room. After a gap of about two weeks, she saw the packet and wanted to do munching again. At that time, her elder sister Pallavi, a science student of class eleven was present in her room. She found that a foul smell was coming out from the packet. She immediately threw it in the dustbin and did not allow her sister to eat the chips.
1. Why did potato chips develop foul smell?
2. What was the cause of the spoilage of the chips?
3. What is the nature of the chemical reaction involved in it?
4. Why do not sealed packets develop foul smell even if kept for months?
5. What values are displaced by Pallavi?
Answer:
1. Potato chips developed foul smell due to rancidity.
2. Potato chips contain some oil as well as fat. These were slowly oxidised since they were exposed to air and therefore, developed foul smell.
3. It is an oxidation reaction
4. The bags or packets containing chips or other such etables are filled with nitrogen and then sealed. This checks rancidity.
5. The knowledge of science came to the help of Pallavi. She was aware of the consequences if one eats rancid food material. She therefore, did not allow her sister to eat the chips and thus, saved her from getting sick.

We hope the above Chemical Reactions and Equations Class 10 Science are useful for you. If you have any questions then post them in the comments section below. Our teachers will provide you an answer. Also refer to MCQ Questions for Class 10 Science


