Please refer to Metals And Non Metals Class 10 Science notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Science books for Class 10. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 10 Science Metals And Non Metals Notes and Questions
Metals are the elements which form positive ions by losing electrons. Thus, metals are known as Electropositive Elements.
Physical Properties of Metals
Metals are the elements that conduct heat and electricity and are malleable and ductile. Examples are Iron (Fe), Aluminum (Al), Silver (Ag), Copper (Cu), Gold (Au), Platinum (Pt), Lead (Pb), Potassium (K), Sodium (Na), Calcium (Ca) and Magnesium (Mg) etc.
Physical Properties of Metals
* Hardness: Most of the metals are hard, except alkali metals, such as sodium, potassium, lithium, etc. are very soft metals. These can be cut by using a knife.
* Strength: Most of the metals are strong and have high tensile strength. Because of this, big structures are made using metals, such as copper (Cu) and iron (Fe). (Except Sodium (Na) and potassium (K) which are soft metals).
* State: Metals are solid at room temperature except for mercury (Hg).
* Sound: Metals produce ringing sound, so, metals are called Sonorous. Sound of metals is also known as Metallic sound. This is the cause that metal wires are used in making musical instruments.
* Conduction: Metals are a good conductor of heat and electricity. This is the cause that electric wires are made of metals like copper and aluminum.
* Malleability: Metals are malleable. This means metals can be beaten into a thin sheet. Because of this property, iron is used in making big ships.
* Ductility: Metals are ductile. This means metals can be drawn into thin wire. Because of this property, a wire is made of metals.
* Melting and Boiling Point: Metals have generally high melting and boiling points. (Except sodium and potassium metals which have low melting and boiling point.)
* Density: Most of the metals have a high density.
* Colour: Most of the metals are grey in colour. But gold and copper are exceptions.
Chemical Properties of Metals
1. Reaction with oxygen: Most of the metals form respective metal oxides when reacting with oxygen.
Metal + Oxygen → Metal Oxide
Examples:
Reaction of Potassium with Oxygen: Potassium metal forms potassium oxide when reacts with oxygen.
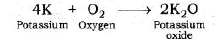
Chemical Properties of Metals
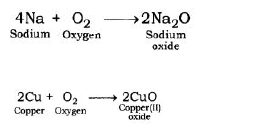
1. Reaction with oxygen: Most of the metals form respective metal oxides when reacting with oxygen.
Metal + Oxygen → Metal Oxide
Examples:
Reaction of Potassium with Oxygen: Potassium metal forms potassium oxide when reacts with oxygen.

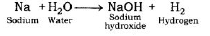
Reaction of Sodium metal with Water: Sodium metal forms sodium hydroxide and liberates hydrogen gas along with lot of heat when reacting with water.
- 3. Reaction of metals with dilute acid: Metals form respective salts when reacting with dilute acid.
Metal + dil. acid → Metal salt + Hydrogen
- Reaction of Sodium metal with dilute hydrochloric acid: Sodium metal gives sodium chloride and hydrogen gas when react with dilute hydrochloric acid.

Hydrogen (H2) gas is not evolved when metal is treated with nitric acid (HNO3):
Nitric acid is strong oxidizing agent and it oxidizes the hydrogen gas (H2) liberated into water (H2O) and itself get reduced to some oxide of nitrogen like nitrous oxide (N2O)3 nitric oxide (NO) and nitrogen dioxide (NO2).
Copper, gold, silver are known as noble metals. These do not react with water or dilute acids.
The order of reactivity of metal towards dilute hydrochloric acid or sulphuric acid is in the order;
K > Na > Ca > Mg > Al > Zn > Fe > Cu > Hg > Ag
AMPHOTERIC OXIDE :Those metal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides. eg. Aluminum oxide and zinc oxide
Reactivity Series of Metals: The order of intensity or reactivity of metal is known as Reactivity Series. Reactivity of elements decreases on moving from top to bottom in the given reactivity series.
In the reactivity series, copper, gold, and silver are at the bottom and hence, least reactive. These metals are known as Noble metals. Potassium is at the top of the series and hence, most reactive.
Reactivity of some metals are given in descending order:
K > Na > Ca > Mg > Al > Zn > Fe > Pb > Cu

Ionic Bonds: Ionic bonds are formed because of transfer of electrons from metal to non-metal.eg. NaCl
Properties of Ionic compound
• Ionic compounds are solid. Ionic bond has a greater force of attraction because of which ions attract each other strongly. This makes ionic compounds solid.
• Ionic compounds are brittle.
• Ionic compounds have high melting and boiling points because force of attraction between ions of ionic compounds is very strong.
• Ionic compounds generally dissolve in water.
• Ionic compounds are generally insoluble in organic solvents; like kerosene, petrol, etc.
• Ionic compounds do not conduct electricity in the solid state.
• The solution of ionic compounds in water conduct electricity. This happens because ions present in the solution of ionic compound facilitate the passage of electricity by moving towards opposite electrodes.
• Ionic compounds conduct electricity in the molten state.
VERY SHORT ANSWER TYPE QUESTIONS
Question. A green layer is gradually formed on a copper plate left exposed to air for a week in a bathroom. What Could this green substance be?
Ans. It is due to the formation of basic copper carbonate (CuCO3.Cu(OH)2.
Question. What is amphoteric oxide? Give examples.
Ans. Those oxides which reacts with acids as well as bases to produce salts and water are called amphoteric Oxides. E.g. Na2O, ZnO etc.
Question. Name the metal which is generally stored under kerosene and easily cut with knife.
Ans. Sodium
Question. Why does calcium float in water?
Ans. It is because hydrogen gas formed which sticks to surface of calcium, therefore it floats.
Question. Name a non-metal which is lustrous and a metal which is non-lustrous.
Ans. Iodine is a non-metal which is lustrous and lead is a non-lustrous metal.
Question. Which gas is liberated when a metal reacts with an acid? How will you test the presence of this gas?
Ans. Hydrogen gas is formed. Bring a burning matchstick near to it, H2 will burn explosively with ‘pop’ Sound.
Question. Name the metal which reacts with a very dilute HNO3 to evolve hydrogen gas.
Ans. Magnesium.
SHORT ANSWER TYPE QUESTIONS
Question. What would you observe when zinc is added to a solution of iron (II) sulphate? Write the chemical Reaction that takes place.
Ans. Zinc is more reactive than iron. When zinc is added to a solution of iron (II) suphate, green color Of iron (II) sulphate fades out and iron metal is deposited.
Zn(s) + FeSO4(aq) —–→ Fe(s) + ZnSO4(aq)
Question. (i) Write the electron-dot structures for sodium and magnesium.
(ii) What are the ions present in Na2O and MgO.
Ans. (i) Electron-dot structures of sodium and magnesium:

(ii) Ion present in Na2O is Na+ and O2-
Ion present in MgO is Mg2+ and O2-
Q3. Why do ionic compound have high melting points?
Ans. Ionic compounds do not exist as single molecules but exist as aggregates of a large number of positive And negative ions due to strong electrostatic forces. Thus, large amount of energy is required to break The inter-ionic attraction, hence these have high melting points.
Q4. Swasti took sulpher powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in the figure.

(a) What will be the action of gas on
(i) Dry litmus paper?
(ii) Moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place?
Ans. (a) (i) no action on dry litmus paper (ii) moist litmus paper becomes re as the reaction taking place.
(b) Balanced equation for the reaction taking place:
S + O2 —-→ SO2
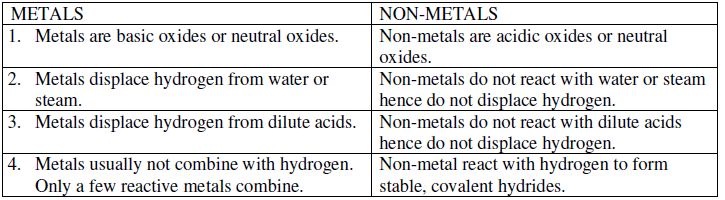
Question. Differentiate between metal and non-metal on the basis of chemical properties.
Ans. Difference between metals and non-metals on the basis of chemical properties.
Question. What is meant by reactivity series of metals? State which of the following chemical reaction will takes Place giving suitable reason for each.
(a) Zn + CuSO4 ——→ ZnSO4 + Cu
(b) Fe + ZnSO4 ——→ FeSO4 + Zn
(c) Zn + FeSO3 ——→ ZnSO4 + Fe
Ans. Reactivity series is a series of metals arranged in the order of their decreasing order of reactivity.
(a) Reaction will take place because Zn is above Cu in the reactivity series and more reactive then Cu.
(b) Reaction will not take place as Fe is below Zn in the reactivity series cannot displace Zn from its Solution.
Question. Using the electronic configuration, explain how magnesium atom combines with oxygen atom to form Magnesium oxide by transfer of electrons.
Ans. Atomic no. of magnesium = 12

LONG ANSWER TYPE QUESTIONS
Question. A non-metal A is the largest constituent of air, when heated with H2 in 1:3 ratio in the presence of catalyst (Fe) gives a gas B. On heating with O2 it gives an oxide C. If this oxide is passed into water in the presence Of air it gives an acid D which acts as a strong oxidizing agent.
(a) Identify A, B, C and D
(b) To which group of periodic table does this non-metal belongs?
Ans. (a) A = N2 Nitrogen
B = NH3Amonia
C = NO Nitrogen
D = HNO3 Nitric oxide
(b) element A belongs to group 15 of the periodic table. Metal
Question. Explain the following:
(a) Reactivity of Al decreases if it is dipped in HNO3.
(b) Carbon cannot reduce the oxides of Na or Mg.
(c) Iron articles are galvanized.
Ans. (a) When Al metal reacts with HNO3, Al2O3 is formed which further gets deposited on aluminum metal. Hence, more Al metal is not available for the reaction because Al2O3 layer is passive in nature.
(b) Na and Mg both metals are very reactive. These metals have more affinity towards oxygen than carbon. Hence, carbon cannot reduce the oxides of these metals.
(c) Galvanization is a process to protect iron from rusting. Iron get rusted when come in contact with air and moisture.
Question. State reason for the following:
(i) Lemon is used for restoring the shine of tarnished copper vessels’
(ii) Copper wires are used in electrical connections.
Ans. (i) when a copper object remains in damp air for a considerable time, the copper reacts slowly with Caron dioxide and water in air to form a green coating of basic copper carbonate on its surface. If corroded copper vessels are treated with lemon which is acidic in nature, the acid solution dissolves Green color basic copper carbonate and makes them look shiny.
(ii)Copper metal is the next best conductor of electricity after silver metal. So electric wires are made of copper.
Question. (a) In the formation of compound between two atoms A and B, A loses two electrons and B gains one Electron.
(i) What is the nature of bond between A and B?
(ii) Suggest the formula of the compound formed between A and B.
(b)On similar lines explain the formation of MgCl2 molecule.
(c)Common salt conducts electricity only in the molten state. Why?
(d)Why is melting point of NaCl high?
Ans. (a)

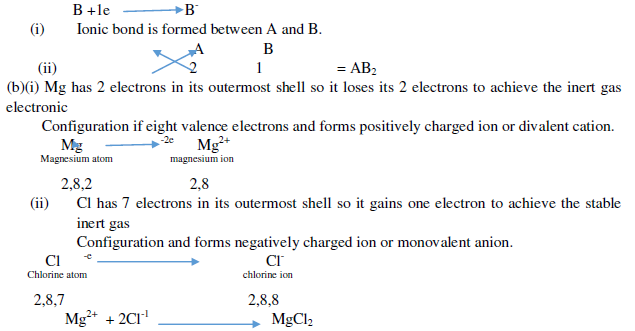
(c)Common salt is an ionic compound which conducts electricity only in molten state because in molten state
The electrostatic forces of attraction between opposite charged ions are overcome due to heat.
Thus ions Move freely and conduct electricity.
(d) NaCl is an ionic compound so there is a strong force of attraction between the positively charged Na Ion and negatively charged chloride ion. Therefore, a considerable amount of energy is required

We hope the above Metals And Non Metals Class 10 Science are useful for you. If you have any questions then post them in the comments section below. Our teachers will provide you an answer. Also refer to MCQ Questions for Class 10 Science


