Students can refer to questions for Class 10 Chemistry Practical Based Questions. The questions are really useful and can help you to get more marks in Class 10 Chemistry Practical exams. All questions and answers provided below have been designed based on the latest Class 10 Chemistry syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should also refer to Class 10 Science notes to get a better understanding of concepts
Class 10 Chemistry Practical Based Questions
GENERAL INSTRUCTIONS:
Read the following instructions very carefully and strictly follow them:
(i) There are 20 questions and all questions are compulsory.
(ii) Questions 1 to 15 are of 1 mark and comprises of multiple-choice questions.
(iii) Questions 16 to 20 are of 2 marks and comprises of short answer questions.
Question 1. Look at the figure given and answer the following: write the reaction involved

a) State the colour of the reactant and the product of the chemical reaction
b) Write the chemical equation involved.
c) Can we convert copper oxide into copper?
Ans. a) Copper powder(reactant) ——– Brown
copper oxide ( product)——-Black
b) 2Cu (s) + O2 heat 2 CuO (s)
c) Yes it can be converted back into copper
CuO (s) + H2(g) ————- Cu (s) + H2O (l)
Question 2. The Chemistry teacher demonstrated an experiment to the students of her class. In her experiment she added an iron nail in a blue colour solution kept in a beaker. After 15 minutes students observed that the blue coloured solution fades.
a) Identify the blue colour solution
b) Name the type of reaction taking place in the beaker
c) Give reason behind decolorization of blue solution. Write the equation involved in the reaction.
Ans. a) Copper sulphate solution CuSO4
b) Displacement reaction
c) Fe (s) + CuSO4 (aq) —– Cu (s) + FeSO4 (aq)
Question 3. Equal length of magnesium ribbon is taken in a test tube A and B. Hydrochloric acid is added to the test tube A, while acetic acid is added to the test tube B. In which test tube will the fizzing occur more vigorously and why?
Ans.In test tube A, freezing occur more vigorously because hydrochloric acid is a strong acid and dissociates more
Question 4. Hari Kiran prepares hydrochloric acid in his school laboratory using certain chemicals. He puts both dry and wet blue Litmus Paper in contact with the gas.
a) Name the reagents used by Hari Kiran to prepare Hydrochloric gas.
b) State the colour changes observed with the dry and wet blue Litmus Paper
c) Show the formation of Ions when hydrochloric gas combines with water.
Ans. a) Sodium chloride and concentrated sulphuric acid
b) There is no colour change observed with dry Litmus Paper. while in wet blue Litmus Paper it turns red
c) HCl (g) + H2O(l) —— H3O+ (aq) + Cl-(aq)
Question 5. A sample of soil is mixed with water and allowed to settle. The clear supinated solution does the pH paper yellow orange. Which of the following would change the colour of this pH paper to greenish blue?
a) Lemon juice
b) Vinegar
c) Common salt
d) An antacid
Ans. (d) An Antacid
Question 6. What does pH of solution signify? Three solutions A, B and C have pH values 6, 2, and 10 respectively. Which of the solution is highly acidic? Which solution will turn red litmus blue?
Ans. pH which measures hydrogen ion concentration in a solution has values 0 to 14. The pH of a neutral solution is 7. Higher the H3O+ ion Concentration, lower the pH value.
Solution with pH value 2 is highly acidic.
Solution with PH value 10 will turn red litmus to blue.
Question 7. Observe the following figures:
Arrange the given test tubes in the increasing order of their pH. justify your arrangement.

Ans. The pH of hydrochloric acid (iii) is the least as it is a strong acid.
The pH of ethanoic acid (I) will be more than (iii) as it is a weak acid.
The pH of water (ii) would be 7.
The pH of sodium bicarbonate(iv)is above 7 as it is a base.
Hence the increasing order of pH is 3, 1 ,2 and 4.
Question 8. Four students were asked to test the pH of 4 samples as shown under. Whose result is reported correctly? Analyse the data.

Ans. Student: D
Question 9. Student at a few drops of universal indicator to three unknown colourless solution P, Q and R taken in three test tubes separately. A student observes the changes in colour as green in P, red in Q and violet in R. arrange the test tube in decreasing order of their pH. Justify the same.
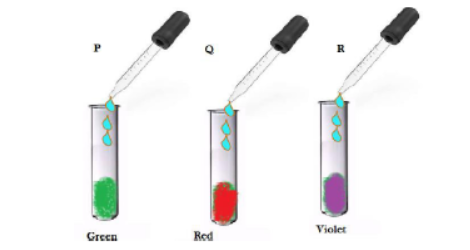
Ans: Arrangement of test tubes in decreasing order of PH would be R > P >Q
R shows violet colour; hence its pH is above 7, P shows green colour, hence its pH is 7, Q shows red colour, hence its pH is 7
Question 10. Which one of the following set-ups is the most appropriate for the evolution of hydrogen gas and its identification? Explain your choice.

Ans: The most appropriate setup is B in this setup the hydrogen gas produced when NaOH is added to zinc granules moves upward due to being lighter than air And is allowed to come in contact with burning matchstick through glass tube in the most proper way.
Question 11. Is is a pH indicator is dipped grape juice What will be the possible colour of pH paper?
a) deep red
b) blue
c) orange
d) violet
Ans: c) orange
Question 12. The two colours seen on the ends and software chart are:
a) Red and blue
c) Red and Green
b) green and blue
d) Orange and green
Ans: a)
Question 13. Hydrogen gas does not show any change with the Litmus paper because:
a) It does not react with Litmus
b) it is neither acidic or basic
c) Both (a) and (b) are correct
d) Both (a) and (b) are incorrect
Question 14. Why does the colour of copper sulphate solution change, when an iron nail is dipped in it?
Ans: On adding iron nail in copper sulphate, displacement reaction will take. Iron being more reactive than copper displaces copper to form a green colour solution of iron sulphate and copper metal is displaced.
Question 15. A student dropped few pieces of marble in dilute hydrochloric acid contained in a test tube. The evolved gas was then passed through lime water. What change would be observed in lime water? Write balanced chemical equation for both the change observed?
Ans: When dilute HCl is added to marble which is calcium carbonate, it forms calcium chloride, water and carbon dioxide. The chemical equation for the reaction is as follows:
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Carbon dioxide gas turns lime water milky due to the formation of calcium carbonate.
The chemical equation showing the reaction between lime water and carbon dioxide is as follows:
Ca (OH)2 + CO2 → CaCO3 + H2O
Question 16. Addition of water in lime is:
a) endothermic reaction c) exothermic reaction
b) Decomposition reaction d) displacement reaction
Ans: exothermic reaction
Question 17. What is the colour of CuSO4. 5H2O?
a) Blue
b) green
c) white
d) yellow
Ans: a)
Question 18. How many water molecules are present in crystals of ferrous sulphate molecules?
a) 5
b) 2
c) 7
d) 10
Ans: c)
Question 19. The reaction of water and quicklime is an example of:
a) combination reaction
b) is thermite reaction
c) Both a) and b)
d) none of these
Ans: c)
Question 20. In double displacement reaction such as the reaction between Sodium sulphate solution and Barium Chloride solution.
A) Exchange of atom take place
B) Exchange of Ions take place
C) A precipitate is produced
D) An insoluble salt is produced
a) (B) and (D)
b) (A) and (C)
c) Only (B)
d) (B) (C) and (D)
Ans: d) When sodium sulphate reacts with barium chloride solution, barium sulphate which is an insoluble product (i.e., precipitate) and sodium chloride are formed.
Question 21. Student strongly heads hydrated ferrous sulphate salt in a dry test tube he would get a
a) Yellow Residue and sweet smell
b) Brown Residue and choking smell
c) Light green Residue and rotten egg smell
d) White Residue and no smell
Ans: b) FeSO4.7H2O ———————– FeSO4 + 2H2O
2FeSO4 ——————-Fe2O3 + SO2 + SO3
Brown residue
Question 22. Arrange sodium magnesium zinc aluminium Copper and gold in order of their decreasing reactivity.
Ans: Na > Mg > Al > Zn > Cu > Au
Question 23. A student takes crystals of ferrous sulphate in a clean and dry test tube. On heating it, what would be the observation?
Ans: Green colour of the ferrous sulphate solution disappears to form a colourless solution and greyish coating of iron is formed on the surface of zinc.
Question 24. A student takes solid Lead Nitrate in a dry test tube and heats it. Give two observations that can be noted.
Ans: White colour Lead Nitrate is decomposed to form yellow colour lead oxide Brown colour gas due to the release of Nitrogen dioxide gas is observed
Question 25. Magnesium ribbon burns in air to form white powder. give the equation to represent the reaction and name the type of reaction.
Ans: 2Mg (s) + O2(g) ———————2MgO (s)
This is called combination reaction
Question 26. Reaction in the lab on mixing radium chloride and sodium sulphate gives white precipitate. Name this kind of reaction and show it by balance chemical equation.
Ans: This kind of reaction is called double displacement reaction and is also called precipitation reaction
Na2SO4 (aq) + BaCl2 (aq) ——–BaSO4 (s) + 2 NaCl (aq)
Question 27. State three types of decomposition reaction:
Ans: three type of decomposition reaction are due to
Heat CaCO3 (s) ——————- CaO (s) + CO2 (g)
Light 2AgBr (s) ——————- 2 Ag (s) + Br2 (g)
Electric current 2H2O ——————— 2H2 (g) + O2(g)
Question 28. What happens when zinc is added to blue colour copper sulphate solution?
Ans: the blue colour copper sulphate solution become colourless.
Question 29. Aluminium sulphate and copper sulphate solutions were taken in two test tubes I and Il respectively. A few pieces of iron filings were then added to both the solutions. The four students A, B, C and D recorded their observations in the form of a table as given below:
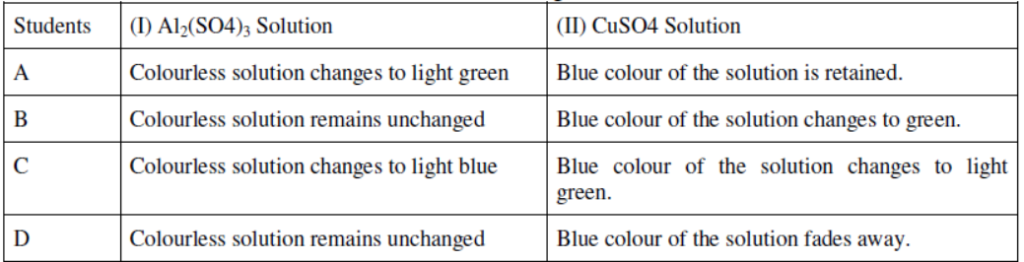
Which student has recorded the correct set of observations.?
Ans: Student B has noted the correct set of observations.
Reason: Fe is less reactive than Al but more reactive than Cu. Hence, displacement reaction will occur in test tube II
Question 30. Which of the following reactions will take place’?
a) Fe + FeSO4
b) Cu + FeSO4
c) Au + FeSO4
d) Mg + ZnSO4
Ans : d)
We hope you liked Class 10 Chemistry Practical Based Questions provided above. Please let us know if you have any questions and problems in Class 10 Chemistry Practical.

