Please refer to Thermal Properties of Matter Class 11 Physics notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Physics books for Class 11. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 11 Physics Thermal Properties of Matter Notes and Questions
The branch dealing with measurement of temperature is called thremometry and the devices used to measure temperature are called thermometers.
Heat
Heat is a form of energy called thermal energy which flows from a higher temperature body to a lower temperature body when they are placed in contact.
Heat or thermal energy of a body is the sum of kinetic energies of all its constituent particles, on account of translational, vibrational and rotational motion.
The SI unit of heat energy is joule (J).
The practical unit of heat energy is calorie.
1 cal = 4.18 J
1 calorie is the quantity of heat required to raise the temperature of 1 g of water by 1°C.
Mechanical energy or work (W) can be converted into heat (Q) by 1 W = JQ
where J = Joule’s mechanical equivalent of heat.
J is a conversion factor (not a physical quantity) and its value is 4.186 J/cal.
Temperature
Temperature of a body is the degree of hotness or coldness of the body. A device which is used to measure the temperature, is called a thermometer.
Highest possible temperature achieved in laboratory is about 108 while lowest possible temperature attained is 10-8 K.
Branch of Physics dealing with production and measurement temperature close to 0 K is knownas cryagenics, while that deaf with the measurement of very high temperature is called pyromet Temperature of the core of the sun is 107 K while that of its surface 6000 K.
NTP or STP implies 273.15 K (0°C = 32°F).
Different Scale of Temperature
1. Celsius Scale In this scale of temperature, the melting point ice is taken as 0°C and the boiling point of water as 100°C and space between these two points is divided into 100 equal parts
2. Fahrenheit Scale In this scale of temperature, the melt point of ice is taken as 32°F and the boiling point of water as 211 and the space between these two points is divided into 180 equal parts.
3. Kelvin Scale In this scale of temperature, the melting pouxl ice is taken as 273 K and the boiling point of water as 373 K the space between these two points is divided into 100 equal pss
Relation between Different Scales of Temperatures
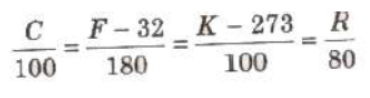
Thermometric Property
The property of an object which changes with temperature, is call thermometric property.
Different thermometric properties thermometers have been given below
(i) Pressure of a Gas at Constant Volume
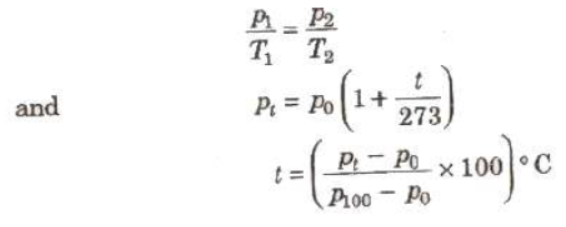
where p, p100. and pt, are pressure of a gas at constant volume 0°C, 100°C and t°C.
A constant volume gas thermometer can measure tempera from – 200°C to 500°C.
(ii) Electrical Resistance of Metals
Rt = R0(1 + αt + βt2)
where α and β are constants for a metal.
As β is too small therefore we can take
Rt = R0(1 + αt)
where, α = temperature coefficient of resistance and
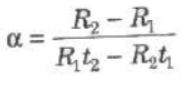
R0 and Rt, are electrical resistances at 0°C and t°C.
where R1 and R2 are electrical resistances at temperatures t1 and t2.
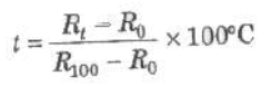
where R100 is the resistance at 100°C.
Platinum resistance thermometer can measure temperature from —200°C to 1200°C.
(iii) Length of Mercury Column in a Capillary Tube
lt = l0(1 + αt)
where α = coefficient of linear expansion and l0, lt are lengths of mercury column at 0°C and t°C.
Thermo Electro Motive Force
When two junctions of a thermocouple are kept at different temperatures, then a thermo-emf is produced between the junctions, which changes with temperature difference between the junctions. Thermo-emf
E = at + bt2
where a and b are constants for the pair of metals.
Unknown temperature of hot junction when cold junction is at 0°C.
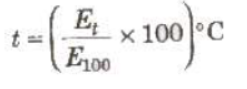
Where E100 is the thermo-emf when hot junction is at 100°C.
A thermo-couple thermometer can measure temperature from —200°C to 1600°C.
Thermal Equilibrium
When there is no transfer of heat between two bodies in contact, the the bodies are called in thermal equilibrium.
Zeroth Law of Thermodynamics
If two bodies A and B are separately in thermal equilibrium with thirtli body C, then bodies A and B will be in thermal equilibrium with each other.
Triple Point of Water
The values of pressure and temperature at which water coexists inequilibrium in all three states of matter, i.e., ice, water and vapour called triple point of water.
Triple point of water is 273 K temperature and 0.46 cm of mere pressure.
Specific Heat
The amount of heat required to raise the temperature of unit mass the substance through 1°C is called its specific heat.
It is denoted by c or s.
Its SI unit is joule/kilogram-°C'(J/kg-°C). Its dimensions is [L2T-2θ-1].
The specific heat of water is 4200 J kg-1°C-1 or 1 cal g-1 C-1, which high compared with most other substances.
Gases have two types of specific heat
1. The specific heat capacity at constant volume (Cv).
2. The specific heat capacity at constant pressure (Cr).
Specific heat at constant pressure (Cp) is greater than specific heat constant volume (CV), i.e., Cp > CV .
For molar specific heats Cp – CV = R
where R = gas constant and this relation is called Mayer’s formula.
The ratio of two principal sepecific heats of a gas is represented by γ.
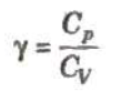
The value of y depends on atomicity of the gas.
Amount of heat energy required to change the temperature of any substance is given by Q = mcΔt
- where, m = mass of the substance,
- c = specific heat of the substance and
- Δt = change in temperature.
Thermal (Heat) Capacity
Heat capacity of any body is equal to the amount of heat energy required to increase its
temperature through 1°C.
Heat capacity = me
where c = specific heat of the substance of the body and m = mass of the body.
Its SI unit is joule/kelvin (J/K).
Water Equivalent
It is the quantity of water whose thermal capacity is same as the heat capacity of the body. It is denoted by W.
W = ms = heat capacity of the body.
Latent Heat
The heat energy absorbed or released at constant temperature per unit mass for change of state is called latent heat.
Heat energy absorbed or released during change of state is given by
Q = mL
where m = mass of the substance and L = latent heat.
Its unit is cal/g or J/kg and its dimension is [L2T-2].
For water at its normal boiling point or condensation temperature (100°C), the latent heat of vaporisation is
L = 540 cal/g
= 40.8 kJ/ mol
= 2260 kJ/kg
For water at its normal freezing temperature or melting point (0°C), the latent heat of fusion is
L = 80 cal/ g = 60 kJ/mol
= 336 kJ/kg
It is more painful to get burnt by steam rather than by boiling was 100°C gets converted to water at 100°C, then it gives out 536 heat. So, it is clear that steam at 100°C has more heat than wat 100°C (i.e., boiling of water).
After snow falls, the temperature of the atmosphere becomes very This is because the snow absorbs the heat from the atmosphere to down. So, in the mountains, when snow falls, one does not feel too but when ice melts, he feels too cold.
There is more shivering effect of ice cream on teeth as compare that of water (obtained from ice). This is because when ice cream down, it absorbs large amount of heat from teeth.
Melting
Conversion of solid into liquid state at constant temperature is melting.
Evaporation
Conversion of liquid into vapour at all temperatures (even below boiling point) is called evaporation.
Boiling
When a liquid is heated gradually, at a particular temperature saturated vapour pressure of the liquid becomes equal to atmospheric pressure, now bubbles of vapour rise to the surface d liquid. This process is called boiling of the liquid.
The temperature at which a liquid boils, is called boiling point The boiling point of water increases with increase in pre sure decreases with decrease in pressure.
Sublimation
The conversion of a solid into vapour state is called sublimation.
Hoar Frost
The conversion of vapours into solid state is called hoar fr..
Calorimetry
This is the branch of heat transfer that deals with the measorette heat. The heat is usually measured in calories or kilo calories.
Principle of Calorimetry
When a hot body is mixed with a cold body, then heat lost by ha is equal to the heat gained by cold body.
Heat lost = Heat gain
Thermal Expansion
Increase in size on heating is called thermal expansion. There are three types of thermal expansion.
1. Expansion of solids
2. Expansion of liquids
3. Expansion of gases
Expansion of Solids
Three types of expansion -takes place in solid.
Linear Expansion Expansion in length on heating is called linear expansion.
Increase in length
l2 = l1(1 + α Δt)
where, ll and l2 are initial and final lengths,Δt = change in temperature and α = coefficient of linear expansion.
Coefficient of linear expansion
α = (Δl/l * Δt)
where 1= real length and Δl = change in length and
Δt= change in temperature.
SuperficialExpansion Expansion in area on heating is called superficial expansion.
Increase in area A2 = A1(1 + β Δt)
where, A1 and A2 are initial and final areas and β is a coefficient of superficial expansion.
Coefficient of superficial expansion
β = (ΔA/A * Δt)
where. A = area, AA = change in area and At = change in temperature.
Cubical Expansion Expansion in volume on heating is called cubical expansion.
Increase in volume V2 = V1(1 + γΔt)
where V1 and V2 are initial and final volumes and γ is a coefficient of cubical expansion.
Coefficient of cubical expansion
where V = real volume, AV =change in volume and Δt = change in temperature.
Relation between coefficients of linear, superficial and cubical expansions
β = 2α and γ = 3α
Or α:β:γ = 1:2:3
2. Expansion of Liquids
In liquids only expansion in volume takes place on heating.
(i) Apparent Expansion of Liquids When expansion of th container containing liquid, on heating is not taken into accoun then observed expansion is called apparent expansion of liquids.
Coefficient of apparent expansion of a liquid
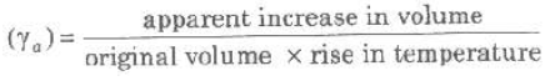
(ii) Real Expansion of Liquids When expansion of the container, containing liquid, on heating is also taken into account, then observed expansion is called real expansion of liquids.
Coefficient of real expansion of a liquid

Both, yr, and ya are measured in °C-1.
We can show that yr = ya + yg
where, yr, and ya are coefficient of real and apparent expansion of liquids and yg is coefficient of cubical expansion of the container.
Anamalous Expansion of Water
When temperature of water is increased from 0°C, then its vol decreases upto 4°C, becomes minimum at 4°C and then increases. behaviour of water around 4°C is called, anamalous expansion water.
3. Expansion of Gases
There are two types of coefficient of expansion in gases
(i) Volume Coefficient (γv) At constant pressure, the change in volume per unit volume per degree celsius is called volume coefficient.
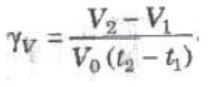
where V0, V1, and V2 are volumes of the gas at 0°C, t1°C and t2°C.
(ii) Pressure Coefficient (γp) At constant volume, the change in pressure per unit pressure per degree celsius is called pressure coefficient.
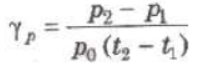
where p0, p1 and p2 are pressure of the gas at 0°C, t1° C and t2° C.
Practical Applications of Expansion
1. When rails are laid down on the ground, space is left between the end of two rails.
2. The transmission cables are not tightly fixed to the poles.
3. The iron rim to be put on a cart wheel is always of slightly smaller diameter than that of wheel.
4. A glass stopper jammed in the neck of a glass bottle can be taken out by warming the neck of the bottles.
Important Points
- Due to increment in its time period a pendulum clock becomes slow in summer and will lose time.
- Loss of time in a time period ΔT =(1/2)α ΔθT
- ∴ Loss of time in any given time interval t can be given by ΔT =(1/2)α Δθt
- At some higher temperature a scale will expand and scale reading will be lesser than true values, so that
- true value = scale reading (1 + α Δt)
- Here, Δt is the temperature difference.
- However, at lower temperature scale reading will be more or true value will be less

We hope the above Thermal Properties of Matter Class 11 Physics are useful for you. If you have any questions then post them in the comments section below. Our teachers will provide you an answer. Also refer to MCQ Questions for Class 11 Physics


