Please refer to Structure of Atom Class 11 Chemistry notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Chemistry books for Class 11. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 11 Chemistry Structure of Atom Notes and Questions
Some Important Points and Terms of the Chapter
1. The word ‗atom‘ has been derived from the Greek word ‘a-tomio‘ which means ‘uncutable‘ or ‘non-divisible‘.
2. J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement (Fig. 2.4, NCERT Page 5). Many different names are given to this model, for example, plum pudding, raisin pudding or watermelon.
3. Rutherford’s Nuclear Model of Atom:
a) Most of the space in the atom is empty as most of the a–particles passed through the foil undeflected.
b) A few positively charged a– particles were deflected. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom as Thomson had presumed. The positive charge has to be concentrated in a very small volume that repelled and deflected the positively charged a – particles.
c) Calculations by Rutherford showed that the volume occupied by the nucleus is negligibly small as compared to the total volume of the atom. The radius of the atom is about 10–10 m, while that of nucleus is 10–15 m.
d) On the basis of above observations and conclusions, Rutherford proposed the nuclear model of atom (after the discovery of protons). According to this model : (i) The positive charge and most of the mass of the atom was densely concentrated in extremely small region. This very small portion of the atom was called nucleus by Rutherford.
(ii) The nucleus is surrounded by electrons that move around the nucleus with a very high speed in circular paths called orbits. Thus, Rutherford‘s model of atom resembles the solar system in which the nucleus plays the role of sun and the electrons that of revolving planets.
(iii) Electrons and the nucleus are held together by electrostatic forces of attraction.
4. The number of protons present in the nucleus is equal to atomic number (Z).the nucleus is equal to atomic number (Z ).i.e. Atomic number (Z) = number of protons in the nucleus of an atom = number of electronsin a nuetral atom
5. Protons and neutrons present in the nucleus are collectively known as nucleons. The total number of nucleons is termed as mass number (A) of the atom. mass number (A) = number of protons (Z) + number of neutrons (n)
6. Isobars are the atoms with same mass number but different atomic number for example, 6C14 and 7N14. On the other hand, atoms with identical atomic number but different atomic mass number are known as Isotopes. e.g. 6C14, 6C13 6C12 & 17Cl35 ,17Cl37
7. Drawbacks of Rutherford Model According to the electromagnetic theory of Maxwell, charged particles when accelerated should emit electromagnetic radiation (This feature does not exist for planets since they are uncharged). Therefore, an electron in an orbit will emit radiation, the energy carried by radiation comes from electronic motion. The orbit will thus continue to shrink. Calculations show that it should take an electron only 10–8 s to spiral into the nucleus. But this does not happen. Thus, the Rutherford model cannot explain the stability of an atom.
8. The frequency (ν ), wavelength (λ) and velocity of light (c) are related by the equation (2.5). c = ν λ The other commonly used quantity specially in spectroscopy, is the wavenumber (ν- ). It is defined as the number of wavelengths per unit length. Its units are reciprocal of wavelength unit, i.e., m–1.
9. H. Hertz performed a very interesting experiment in which electrons (or electric current) were ejected when certain metals (for example potassium, rubidium, caesium etc.) were exposed to a beam of light . The phenomenon is called Photoelectric effect. For photoelectric effect : hv0 + 1/2 mu2
10. Planck’s quantum theory. (i) The energy is radiated or absorbed by a body not continuously but discontinuously in form of small packets.
(ii) Each packet is called quantum. In case of light, the quantum is called ‘photon‘. The energy of quantum is directly proportional to the frequency (v) of the radiation. E ∝ v E = hv, Where “h” is Planck‘s constant. Its value is 6.625 X 10-34 Joule second.
11. The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum. Atoms, molecules or ions that have absorbed radiation are said to be ― excited‖. To produce an emission spectrum, energy is supplied to a sample by heating it or irradiating it and the wavelength (or frequency) of the radiation emitted, as the sample gives up the absorbed energy, is recorded.
12. An absorption spectrum is like the photographic negative of an emission spectrum. A continuum of radiation is passed through a sample which absorbs radiation of certain wavelengths.
13. Line Spectrum of Hydrogen: When an electric discharge is passed through gaseous hydrogen, the H2 molecules dissociate and the energetically excited hydrogen atoms produced emit electromagnetic radiation of discrete frequencies. The hydrogen spectrum consists of several series of lines named after their discoverers.

The Swedish spectroscopist, Johannes Rydberg, noted that all series of lines in the hydrogen spectrum

could be described by the following expression :
14. Bohr’s Model For Hydrogen Atom
a) The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states. These orbits are arranged concentrically around the nucleus.
b) An electron can move only in those orbits for which its angular momentum is integral multiple of h/2Π that is why only certain fixed orbits are allowed. The angular momentum of an electron in a given stationary state can be expressed as in equation
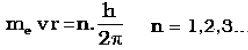
c) The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state . The energy change does not take place in a continuous manner.
d) The frequency of radiation absorbed or emitted when transition occurs between two stationary states that differ in energy by ΔE, is given by :

15. Bohr‘s theory can also be applied to the ions containing only one electron, similar to that present in hydrogen atom. For example, He+ Li2+, Be3+ and so on. The energies of the stationary states associated with these kinds of ions (also known as hydrogen like species) are given by the expression
16. Limitations of Bohr’s Model: It fails to account for the finer details (doublet, that is two closely spaced lines) of the hydrogen atom spectrum observed by using sophisticated spectroscopic techniques. This model is also unable to explain the spectrum of atoms other than hydrogen, for example, helium atom which possesses only two electrons. Further, Bohr‘s theory was also unable to explain the splitting of spectral lines in the presence of magnetic field (Zeeman effect) or an electric field (Stark effect).
17. Dual Behaviour of Matter: The French physicist, de Broglie in 1924 proposed that matter, like radiation, should also exhibit dual behaviour i.e., both particle and wavelike properties.
18. The de Broglie relation. :de Broglie relation state that the wavelength associated with a moving object or an electron is inversely proportional to the momentum of the particle.
λ = h/m = h/p where p is the momentum of particle = mv.
19. Heisenberg’s Uncertainty Principle. It is not possible to determine the position and velocity simultaneously for a sub-atomic particle like electron at any given instant to an arbitary degree of precision. Consequently, it is not possible to talk of path of the electron in which it moves. If ‘Δ x‘ is uncertainty in position and ‘Δ P‘ is uncertainty in momentum then ΔxΔP≥ (h/4π)
20. Orbital. It is a region or space where there is maximum probability of getting electron.
21. Quantum numbers. They are used to get complete information about electron, i.e., location,
energy, spin, etc. These quantum numbers also help to designate the electron present in an
orbital.
22. Principal quantum number. It specifies the location and energy of an electron. It is measure of the effective volume of the electron cloud. It is denoted by ‘n‘. Its possible values are 1, 2,
3,4 …..
23. Angular momentum quantum number. It is also called ‗azimuthal quantum number‘. It determines the shape of the orbital. It is denoted by ‘l‘. The permitted values of ‘l‘ are 0, 1, 2, etc., up to n–1. For a given value of n, l = 0 to n – 1 ‘. e.g., if value of n is 4, l can have values 0, 1, 2, 3. It determines angular momentum.
24. Magnetic quantum number. It is denoted by ‘m‘ and its value depends on value of ‘l‘ since magnetism is due to angular momentum. It determines the magnetic orientation of an orbital, i.e., the direction of orbital relative to magnetic field in which it is placed. Its permitted values are – l to + l including zero, e.g., when l = 1, then m = -1, 0, +1. It has total number of values equal to 2l + 1.
25. Spin quantum number. It indicates, the direction in which electron revolves. Spin is magnetic property and is also quantized. It has two permitted values + ½ or – ½. The spin angular momentum of an electron is constant and cannot be changed.
26. (n + ) rule: The relative order of energies of various sub-shells in a multi-electron atom can be predicted with the help of (n + l) rule (also called Bohr-Bury rule)According to this rule a subshell with lower values of (n + l) has lower energy. In case two sub-shell has equal value of (n +l ), the sub-shell with lower value of n has lower energy
27. Pauli’s Exclusion Principle. No two electrons in an atom can have all the four quantum numbers same. It can also be stated as – An orbital can have maximum two electrons and they must be of opposite spin quantum numbers.
28. Aufbau principle. Electrons are filled in the various orbitals in the increasing order of their energies, i.e., orbital having lowest energy will be filled first and the orbital having highest energy will be filled last. Increasing energy of atomic orbitals for multi-electron atoms 1s < 2s < 2p < 3s <3p < 4s <3d < 4p <5s < 4d <5p < 6s < 4f < 5d < 6p < 7s
29. Hund’s rule of maximum multiplicity. No electron pairing takes place in p, d and f orbitals until each orbital in the given sub-shell contains one electron, e.g., N (7) has electronic configuration 1s2 2s2 2p1x 2p1y 2p1z according to Hund‘s rule and not 1s2 2s2 2px2 2py1.
30. The valence electronic configurations of Cr and Cu, therefore, are 3d5 4s1 and 3d104s1 respectively and not 3d4 4s2 and 3d94s2. It has been found that there is extra stability(Stability of Completely Filled and Half Filled Subshells) associated with these electronic configurations.
31. Three orbitals of 2p subshell (2px, 2py, and 2pz orbitals).

Five orbitals of 3d subshell (3dxy, 3dyz, 3dzx 3dx2-y2 and 3dx2 orbitals).




