Please refer to The p Block Elements Class 11 Chemistry notes and questions with solutions below. These revision notes and important examination questions have been prepared based on the latest Chemistry books for Class 11. You can go through the questions and solutions below which will help you to get better marks in your examinations.
Class 11 Chemistry The p Block Elements Notes and Questions
Elements in which the last electron enters in the any one of the three p- orbital of their outermost shells – p-block elements
• Gen. electronic configuration of outer shell is ns2np1-6
The inner core of e-config. may differ which greatly influences their physical & to some extent chemical properties.
• The block of elements in the periodic table consisting of the main groups :
• Group 13 (B to Tl)
• Group14 (C to Pb)
• Group15 (N to Bi)
• Group 16 (O to Po)
• Group17 (F to At)
• Group18 (He to Rn)
(1) Members at the top and on the right of the p-block are nonmetals (C, N, P, O,F, S, Cl, Br, I, At).
(2) Those on the left and at the bottom are metals (Al, Ga, In,Tl, Sn, Pb, Sb Bi, Po).
(3) Between the two, from the top left to bottom right, lie an ill-defined group of metalloid elements (B, Si, Ge, As, Te)
Group 13 : The boron group
• Outer Electronic Configuration:-ns2np1
• group members: boron (B), aluminum (Al), gallium (Ga), indium (In) & thallium (Tl) . All, except boron, are metals.
• Boron show diagonal relationship with Silicon; both are semiconductors metalloids & forms covalent compounds.
• Boron compounds are electron deficient, they are lack of an octet of electrons about the B atom .
• diborane B2H6, is simplest boron hydride
• Structure: three-center two-electron: the H atoms are simultaneously bonded to two B atoms the B-H bridging bond lengths are greater than B-H terminal.
• – Boron oxide is acidic (it reacts readily with water to form boric acid)
• aluminium compounds: aluminium oxide is amphoteric
• aluminum halides, e.g., AlCl3 is dimer, an important catalyst in organic chemistry have anin complete octet, acts as Lewic acid by accepting lone pairs from Lewic bases, forming adduct
• aluminum hydride, e.g., LiAlH4, a reducing agent
• Atomic Properties – Electronic Configurations
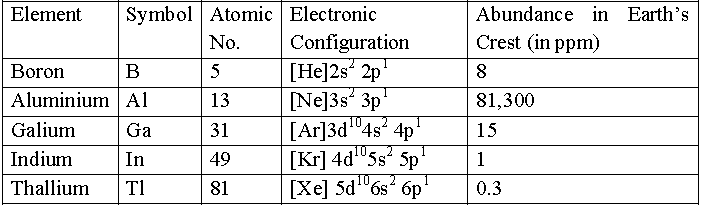
Atomic and ionic radii
• The atomic and ionic radii of group 13 elements are compared to corresponding elements of group 2. From left to right in the period, the magnitude of nuclear charge increases but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge.
• In other words, effective nuclear charge increases and thus, size decreases. Therefore, the elements of this group have smaller size than the corresponding elements of second group.
• On moving down the group both atomic and ionic radii are expected to increase due to the addition of new shells. However, the observed atomic radius of Al (143 pm) is slightly more than that of Ga (l35 pm).
Ionization energies
The first ionization energies of group 13 elements are less than the corresponding members of the alkaline earths.
The sharp decrease in I.E. from B to Al is due to increase in size. In case of Ga, there are ten d-electrons in its inner electronic configuration.
The very high value of 3rd I. E. of thallium indicates that +3 O.N. state is not stable, rather +1 is more stable for thallium.
Electropositive (or metallic) character
the elements of group 13 are less electropositive as compared to elements of group 2. On moving down the group the electropositive (metallic) character increases because ionization energy decreases. For e.g., Boron is a non-metal white the other elements are typical metals.
Oxidation states
The common oxidation states of group 13 elements are +3 and + l .The stability of the + 1 oxidation state increases in the sequence Al <Ga< In <Tl, Due to Inert pair effect.

Chemical reactivity of Gr.13 Elements
All elements in their compounds exhibit the oxidation state of + 3 and +1.
Hydrides
• None of the group 13 elements reacts directly with hydrogen. However, a no. of hydrides of these elements have been prepared by indirect methods. The boron hydrides are called boranes& classified in two series: (a) BnHn+4 called nidoboranes (b) BnHn+6 called arachnoboranes
• Inudustrial Preperation :-
2BF3(g) + 6LiH(s) → B2H6(g) + 6LiF(s)
• Laboratory method:
(i) By the reaction of iodine with sodium borohydride in a high boiling solvent.
2NaBH4 + I2 → B2H6 + 2NaI + H2
(ii) By reduction of BCl3 with LiAlH4
4BCl3 + 3LiAlH4 → 2 B2H6 + 3AlCl3 + 3 LiCl
Structure of Diborane, B2H6

Some important characteristics of boranes:
i) Lower boranes are colour less gases while higher boranes are volatile liquids or solids.
ii) They undergo spontaneous combustion in air due to strong affinity of boron for oxygen.
B2H6 + 3O2 → B2O3 + 3H2O + Heat
iii) Boranes react with alkali metal hydrides in diethyl ether to form borohydride complexes.
B2H6 + 2MH →2M+[BH4]– (M= Li or Na)
Metal borohydride
• (iv) Diborane reacts with ammonia to give borazine at 450 K.
B2H6 + 6NH3 → 3B3N3H6 + 12H2
• Borazine has a cyclic structure similar to benzene and thus is called inorganic benzene
• The other elements of this group form only a few stable hydrides. The thermal stability decreases as we move down the group.
• AlH3 is a colour less solid polymerized via Al – H – Al bridging units. These hydrides are weak Lewis acids and readily form adducts with strong Lewis base (B:) to give compounds of the type MH3 (M = Al or Ga). They also form complex-tetrahydrido anions, [MH4]-. The most important tetrahydrido compound is Li[AlH4]
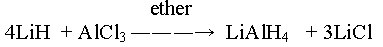
Oxides & Hydroxides
• M2O3& M(OH)3
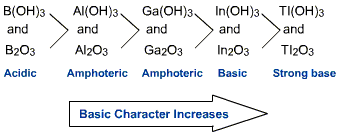
Halides : Structure of boron trihalides

Dimeric structure of aluminium chloride – Boron halides do not form dimers because the size of boron is so small that it is unable to coordinate four large-sized halide ions.

• Anomalous properties of boron
1. Boron is a non-metal & bad conductor of electricity whereas aluminium is a metal & good conductor. B is hard but Al is a soft metal.
2. Boron exists in two forms-crystalline and amorphous. But Al does not exist in different forms.
3. The melting and boiling point of boron are much higher than that of Al .
4. Boron forms only covalent compounds whereas Al forms even some ionic compounds.
5. The hydroxides and oxides of boron are acidic in nature whereas those of aluminium are amphoteric.
6. The trihalides of boron exist as monomers. On the other hand, aluminium halides exist as dimers .
7. The hydrides of boron are quite stable while those of aluminium are unstable
• Boron and silicon exhibit the typical properties of non-metals. These do not form cations. Both exist in amorphous as well as crystalline forms.
• Boron oxide (B2O3) and silica (SiO2) both are acidic and dissolve in alkali solutions to form borates and silicates respectively.
B2O3 + 6NaOH → 2Na2BO3 + 3H2O
SiO2 + 2NaOH → Na2SiO3 + H2O
• The chlorides of both B and Si get hydrolyzed by water to boric acid and silicic acid respectively.
BCl3 + 3H2O →H3BO3 + 3HCl SiCl4 + 3H2O → H2SiO3 + 4HCl
The hydrides of Boron and Silicon are quite stable. Numerous volatile hydrides are also known which catch fire on exposure to air and are easily hydrolyzed.
• Both elements are semiconductors.
Behavior in Aqueous Solutions
1 Al, Ga, In and Tl exhibit a well-defined aqueous chemistry in their tripositive states. Species like [M(OH)4]-, [M(H2O)2(OH)4]-, [M(OH2)6]3+ for M = Al, Ga, In, exist in aqueous solution.
2. Al, Ga. In and T1 ions exist as octahedral aqua ions, [M(OH2)6]3+ in aqueous solution and many salts like halides, sulphates, nitrates and perchlorates exist as hydrates.
3. Aluminium sulphate forms double salts – called alum, having the general formula M2SO4. Al2(SO4)3.12H2O, where M=Na+ or K+.
Uses Of Boron & Aluminium
• Aluminium is used extensively in industry and everyday life. It forms many useful alloys with Cu. Mn, Mg, Si and Zn. Hence, aluminium and its alloys find use in packaging, utensil making, construction, aerospace and other transportation industries. It is used as a conductor for transmission of electricity. Aluminium is alsoused in the alumino-thermite process for production of chromium and manganese from their ores.
Group 14 Elements:-The Carbon Family
Group 14 includes carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb).
General electronic configuration of carbon family is ns2np2.
Covalent radius:-Covalent radius expected to increase from Cto Si, From Si to Pb small increase is found.
Ionization Enthalpy:- The first ionization enthalpies of group 14 elements are higher than those of the corresponding group 13 elements.
Electronegativity:- Group 14 elements are smaller in size as compared to group 13 elements that’s why this group elements are slightly more electronegative than group 13
Chemical properties:-
Carbon and silicon mostly show +4 oxidation state. Germanium forms stable compounds in +4 state and only few compounds in +2 state.
Tin forms compounds in both oxidation states. Lead compounds in +2 state are stable and in +4 state are strong oxidizing agents.
Exception:- Pb4 and SnF4 are ionic in nature.
Except CCl4 other tetrachloride’s are easily hydrolysed by water.
Since carbon does not have d-orbitals and hence cannot expand its coordination number beyond 4
CCl4 +H2O No Reaction
SiCl4+4H2O Si(OH)4+4HCl
Silicic acid
Allotropes of Carbon:-The three types of allotropes are –
1- Diamond 2 – Graphite 3 – Fullerence
Diamond:- In diamond each carbon atom under gas SP3hybridisation.
Each carbon is tetrahedrally linked to four other carbon atoms.
Graphite:- In graphite, carbon is SP2-hyberdized graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together.
Graphite conducts electricity along the sheet. It is very soft and Slippery
Fullerence :- Fullerence was discovered collectively by three scientists namely R.E Smalley, R.F Curl and H.W K roto
Some Important Compounds Of Carbon and Silicon
Carbon monoxide:- It I prepared by direct oxdisation of C in limited supply of oxygen.
2C+O2(g) → 2CO(g)
Laboratory method:-
In laboratory it is prepared by the treatment of dil HCl on CaCO3
CaCO3(s) +2HCl(aq) → CaCl2(aq) +CO2(g)+H2O(l)
Silicon dioxide :- Silicon dioxide is a Covalent Three Dimensional
Network Solid.
Each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms.
Silicones :- Silicones are the synthetic organo-silicon polymers having general formulae (R2SiO)n in which R = alkyl (methyl, ethyl or phenyl)
Silicates :- Silicates are exist in nature in the form of feldspar, zeolites, mica and asbestos etc.
The basic structure of silicates is SiO44-
Zeolites:-Zeolites is aalumino-silicate of metal. Metal cations participating in formation of Zeolite are use usually Na+,K+,or Ca2+.
Zeolites are used to remove permanent hardness of water.
Question. Why is boron used in nuclear reactions?
Ans. Because Boron can absorb neutrons.
Question. By giving a balanced equation show how B(OH)3 behaves as an acid in water.
Ans. B(OH)3 +2HOH → [B(OH)4]– +H3O+
Question. Name the element of group 14 which exhibits maximum tendency for catenation?
Ans. Carbon
Question. What is the basic building unit of all silicates?
Ans. SiO4
4-is the basic unit of all silicates.
Question. What happens when NaBH4 reacts with iodine?
Ans. 2NaBH4 +I2 → B2H6 +2NaI +H2-
Question. What happens when boric acid is heated
Ans. 4H3BO3 → 4HBO2 H2B4OQuestion.
Question. What is producer gas?
Ans. Producer gas is a mixture of CO and N2 in the ratio of 2:1
Question. Write the state of hybridization of ‘B’ in BF3.
Ans. Hybridisation of ‘B’ in BF3 is Sp2.
Question. Mention the state of hybridization in B in BH4–.
Ans. Sp2.
Question. Which oxide of carbon is regarded as anhydride of carbonic acid.
Ans. CO2 is regarded as a hydride of carbonic acid .
Because H2CO3 → H2O + CO2
Question. Give the chemical reaction as an evidence for each of the following observations.
(i) Tin (II) is a reducing agent where as lead (II) is not.
(ii) Gallium (I) undergoes disproportionation reaction.
Ans. (i) Due to inert pair effect pb2+ is more stable than Pb4+. Whereas Sn4+ is more stable than Sn2+.
(ii) 3Ga+ → 2Ga +Ga3+
This is because Ga3+ is more stable than Ga+.
Question. What happens when
(i) Quick lime is heated with coke?
(ii) Carbon monoxide reacts with Cl2
Ans. (i) Cao +3C → CaC2 +CO
(iii) CO +Cl2→ COCl2
Question. Give reason
(i) C and Si are always tetravalent but Ge, Sn, Pb show divalency.
(ii) Gallium has higher ionization enthalpy than Al. Explain.
Ans. (i) Ge, Sn, Pb show divalency due to inert pair effect, Pb2+ is more stable than Pb4+.
(ii) Due to poor shielding effect of d-electrons in Ga effective nuclear charge increases as compared to Al thus the I.E is higher than Al.
Question. Give reason why boron and aluminium tend to form covalent compounds.
Ans. Sumof three ionization of both the element are very high. Thus they have no tendency to lose electrons to form ionic compound .Instead they form covalent compounds.
Question. If B-Cl bond has a dipole moment, Explain why BCl3 molecule has zero dipole moment.
Ans. B-Cl bond has dipole moment because of polarity.In BCl3 since the molecule is symmetrical thus the polarities cancel out.
Question. Suggest a reason as to why CO is poisonous.
Ans. CO reacts with haemoglobin to form carboxy-haemoglobin which can destroy the oxygen carrying capacity of haemoglobin and the man dies of suffocation.
Question. What do you understand by-
(a) Inert pair effect:- The pair of electron in the valence shell does not take part in bond formation it is called inert pair effect.
(b) Allotropy :- It is the property of the element by which an element can exists in two forms which have same chemical properties but different physical properties due to their structures.
Question. How is excessive content of CO2 responsible for global warming?
Ans. Excess of CO2 absorbs heat radiated by the earth. Some of it dissipated into the atmosphere while the remaining part is radiated back to the earth. Temperature of the earth increases.
Question. Describe two similarities and two dissimilarities between B and Al.
Ans. Similarities:-
(i) Both have same number of valence electrons.
(ii) Both have similar electronic configuration.
Dissimilarities:-
(i) Bis a non- metal where Al is a metal
(ii) B forms acidic oxide whereas Al forms atmospheric oxides.
Question. What are fullerene? How they were prepared?
Ans. Fullerene are the allotropes of carbon. Its structure is like a soccer ball.
They are prepared by heating graphite in electric arc in presence of inert gases such as helium or argon.
Question. What happens when
(a) Borax is heated strongly
(b) Boric acid is added to water
(c) Aluminium is treated with dilute NaOH
Ans. (a) Na2B4O7 . 10H20 → Na2B4O7→ 2NaBO2 + B2O3
(b) B(OH)3 +H2O → [B(OH)4]- +H+
(C) 2Al +2NaOH +H2O → 2NaAlO2 + 3H2
Question. Explain the following reactions.
(a) Silicon is heated with methyl chloride at high temperature in the presence of copper.
(b) Silicon dioxide is treated with hydrogen fluoride.
(c) CO is heated with ZnO.
Ans. (a) A mixture of mono-,di- and trimethyl chlorosilianes along with a small amount of tetramethylsilane is formed.
CH3Cl +Si → CH3SiCl3 + (CH3)2 SiCl2+(CH3)3SiCl +(CH3)4 Si
(b) The initially formed silicon tetrafluroide dissolves in HF to form ydrofluorosilicic acid
SiO2 +2HF → SiF4 +2H2O
SiF4 + 2HF → H2SiF6
(c) ZnO is reduced to zinc metal
ZnO + CO → Zn +CO2
Question. Give reasons:-
(a) Diamond is used as an abrasive.
(b) Aluminium alloys are used to make aircraft body.
(c) Aluminium utensils should not be kept in water overnight.
Ans. (a) Diamond is used as an abrasive because it is an extremely hard substance.
(b) Alloys of alumimium like duralium is used to make aircraft body due to Some of its property.
(c) Generally aluminium metal does not react with water quickly but when it is kept overnight. It reacts slowly with water in presence of air.
2Al(s) +O2(g) +H2O(l) → Al2O3(s) +H2(g)
Question. A certain salt X, gives the following results.
(i) Its aqueous solution is alkaline to litmus.
(ii) It swells up to a glassy material Y on strong heating.
(iii) when conc. H2SO4 is added to a hot solution of X, white crystal of an acid Z separates out.
Ans. (i) Na2B4O7 +10H2O → 2NaOH +H2B4O7 + 8H2O
(ii) Na2B4O7 → 2NaBO2 +B203
(iii) Na2B4O7 .10H2O + H2SO4 → 4H3BO3 +Na2SO4 +5H2O
Question. draw structure of diborane .
Ans.
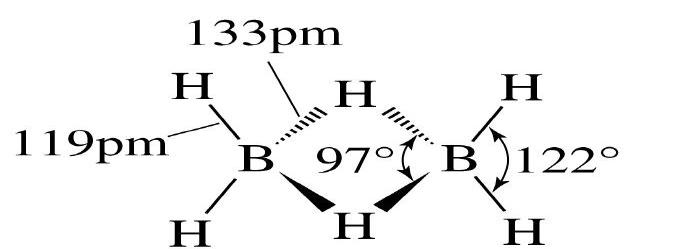
1 Explain the formation of (i) Water gas (ii) Producer gas. Give their uses. What happens when CO2 is passed through lime water (i) for short duration (ii) folong duration.
Ans. (i) C(s) + H2O(g) → CO(g) +H2(g) (Water gas)
(ii) 2C(s) + O2 +4N2(g) → 2CO(g) +4N2(g)
(Producer gas)
Water gas and Producer gas are used as fuel.
Ca(OH)2 +CO2→ CaCO3 + H2O
(White ppt.)
(i) CaCO3 +CO2 +H2O →Ca(HCO3)2
(Soluble)
Question. (a) Why do Boron halides from addition compound with NH3 ?
(b) Assign appropriate reason for each of the following observations :-
(i) Anhydrous AlCl3 is used as a catalyst in many organic reactions.
(ii) No form of elemental silicon is comparable to graphite.
Ans. (a) It is because BX3 is electron deficient whereas NH3 is electron rich.
(b) (i) It is Lewis acid.
(ii) It cannot form pπ – pπ bond due to large size.
Question. (i) Give reason for the following observations:-
(a) The tendency for catenation decreases down the group in Group 14.
(b) The decreasing stability of +3 oxidations state with increasing atomic number in group 13.
(c) PbO2 is a stronger oxidizing agent than SnO2.
(d) Molten aluminium bromide is a poor conductor of electricity.
Ans. (i) (a) It is due to decrease in bond dissociation energy which is due to increase in atomic size.
C-C > Si-Si >Ge-Ge>Sn-Sn>Pb-Pb.
(b) It is due to inert pair effect.
(c) PbO2 is stronger oxidizing agent than SnO2 because Pb2+ is more stable than Pb4+ whereas Sn4+ is more stable than Sn2+.
(d) Molten AlBr3 is poor conductor of electricity because it is covalent compound.