Please refer to Class 10 Science Sample Paper Set C below provided with solutions. All Class 10 Science Sample Paper have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS. All questions have been provided with solutions.
Class 10 Science Sample Paper Set C
Time: 3 Hours Maximum Marks: 80
General Instructions:
(i) The question paper comprises four sections A, B, C and D. There are 36 questions in the question papers. All questions are compulsory.
(ii) Section–A – question no. 1 to 20 – all questions and parts thereof are of one mark each. These questions contain multiple choice questions (MCQs), very short answer questions and assertion – reason type questions. Answers to these should be given in one word or one sentence.
(iii) Section–B – question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should in the range of 30 to 50 words.
(iv) Section–C – question no. 27 to 33 are short answer type questions, carrying 3 marks each. Answers to these questions should in the range of 50 to 80 words.
(v) Section–D – question no. – 34 to 36 are long answer type questions carrying 5 marks each. Answer to these questions should be in the range of 80 to 120 words.
(vi) There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
(vii) Wherever necessary, neat and properly labeled diagrams should be drawn
SECTION – A
Question 1. Define the term malleability and ductility (1 Marks)
OR
Why does calcium float in water
Answer. Malleability – material is one in which a thin sheet can be easily formed by hammering or rolling
Ductility – It is the property of metals by which metals can be drawn into thin wires
OR
Calcium reacts with water to form calcium hydroxide and hydrogen gas which form bubbles and stick to the surface of calcium metal making it light due to which calcium floats on water
Question 2. What is meant by a saturated hydrocarbon? (1 Marks)
Answer. Those hydrocarbons in which valency of carbon is satisfied by single bonds only are called saturated hydrocarbons.
Question 3. Which Salt from the following is used to cure irritation and pain due to the in digestion and why? (1 Marks)
(a) Caustic soda
(b) Washing soda
(c) Baking soda
(d) Simple salt
Answer. Baking soda. It neutralizes effect of acid in stomach
Question 4. How does the sky appear from the surface of the moon? (1 Marks)
Answer. The sky appears dark from the surface of the moon because there are no atmospheric particles to scatter sunlight
Question 5. When is real image formed by the light rays after reflection or refraction? (1 Marks)
Answer. A real image is formed when the rays of light after reflection or refraction actually meet at some point
Question 6. Define power of lens. Name its unit. (1 Marks)
OR
Where should an object be placed in front of a convex lens to get a real image of the same size of the object
Answer. The ability to converge or diverge the ray of light falling on it. Unit of power is Diopter (D)
OR
At twice the focal length
Question 7. What is the nature of the magnetic field associated with a straight current carrying conductor? (1 Marks)
Answer. The magnetic lines of force are concentric circles with the conductor
Question 8. What is the value magnetic field inside a current carrying solenoid ? (1 Marks)
Answer. It is uniform
Question 9. What is the power of a bulb rated at 2.5 V and 0.5 A (1 Marks)
OR
What is mean by saying that potential difference between two point is 1 volt ?
Answer. P=VI 2.5X.05= 1.25watt
OR
1 joule of work is done in moving 1coulomb of electric charge from one point to other point.
Question 10. Name the term for transport of food from leaves to other parts of the plant. (1 Marks)
Answer. Translocation of food
Question 11. Describe excretion in organisms? (1 Marks)
OR
Define cellular respiration.
Answer. The biological process of removal of harmful nitrogenous metabolic waste from the body is called excretion.
OR
The process by which organisms use oxygen to break down food molecules to get chemical energy for cell functions
Question 12. Ozon is deadly poisonous, still it performs an essential function. How ? (1 Marks)
OR
State any two environment-friendly practices.
Answer. it is essential because it shields the surface of the earth from the entry of UV rays from the sun which can harm us by causing skin burnings and skin cancer
Question 13. How do plants get carbon dioxide for photosynthesis ? (1 Marks)
For question numbers 14, 15 and 16, two statements are given- one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both A and R are true, and R is correct explanation of the assertion.
(b) Both A and R are true, but R is not the correct explanation of the assertion.
(c) A is true, but R is false.
(d) A is false, but R is true.
Answer. By animals through stomata
Question 14. Assertion (A): carbon has an extremely large number of compounds (1 Marks)
Reason (R): the chain of carbon atom act as backbone to which another atom and functional group can be attached
Answer. (a) Both A and R are true, and R is correct explanation of the assertion.
Question 15. Assertion (A): clear sky appears blue. (1 Marks)
Reason (R): blue color of light has smaller wavelength, so it scatters more in upper layer of atmosphere in comparison to the other layers.
OR
Assertion (A): rainbow always appear on the same side as the sun
Reason (R) : rainbow is natural spectrum which occurs after a shower
Answer. (c) A is true, but R is false.
OR
(d) A is false, but R is true.
Question 16. Assertion (A): basic event in reproduction is the creation of a DNA copy (1 Marks)
Reason (R): cell use chemical reactions to build copies of their DNA
Answer. (b) Both A and R are true, but R is not the correct explanation of the assertion.
| Answer Q. No 17 – 20 contain five sub-parts each. You are expected to answer any four sub-parts in these questions. |
Question 17. Read the following passage and answer any four question from 17(a) to 17(e). (1× 4 marks)
Human body is made up of five important components, of which water is the main component. Food as well as potable water are essential for every human being. The food is obtained from the plants through agriculture. Pesticides are being used extensively for a high yield in the fields. These pesticides are absorbed by the plant from the soil along with the water and Minerals and from the water bodies these pesticides are taken up by the aquatic animals and plants. As these Chemicals are not biodegradable, they get accumulated progressively at each trophic level. The maximum concentration of these Chemicals gets accumulated in our bodies and greatly affected the health of our mind and body.
(a) The maximum concentration of pesticides is found in human beings because humans are at the
(i) highest trophic level
(ii) first trophic level
(ii) second trophic level
(iv) producer level
Answer. (i) highest trophic level
(b) Our intake of pesticides through food can be reduced to some extent by
(A) encouraging organic farming
(B) using herbicides
(C) using biopesticides
(D) using fertilizers
(i) A, B and D
(ii) A,C and D
(iii) A, B and C
(iv) A,B,C and D
Answer. (iii) A, B and C
(c) Various steps in a food chain represent
(i) food web
(ii) trophic level
(iii) ecosystem
(iv) biomagnification
Answer. (ii) trophic level
(d) With regard to various food chain operating in ecosystem man is a
(i) consumer
(ii) producer
(iii) producer and consumer
(iv) producer and decomposer
Answer. (i) consumer
(e) The plants are __________ in ecosystem
(i) consumer
(ii) producer
(iii) producer and consumer
(iv) producer and decomposer
Answer. (ii) producer
Question 18. Read the following table and answer any four question from 18(a) to 18(e) (1× 4 marks)

(a) An insoluble base among the following is
(i) A
(ii) B
(iii) C
(iv) D
Answer. (iii) C
(b) Substance D can be
(i) baking soda
(ii) battery acid
(iii) milk of magnesia
(iv) ammonia
Answer. (ii) battery acid
(c) A farmer found that the pH of soil in his field is 4.2. Any two substances from the given table which he should be
(i) A and B
(ii) C and D
(iii) E and F
(iv) (ii) and (iii) both
Answer. (iv) (ii) and (iii) both
(d) If the pH increases from 7 to 14 what does it shows in terms of OH– ion concentration?
Answer. OH– ion concentration is increasing in the solution.
(e) The tooth enamel starts corrode when the pH becomes
(i) <5.5
(ii) > 5.5
(iii) < 5
(iv) < 6
Answer. (ii) > 5.5
Question 19. Read the following table and answer any four question from 19(a) to 19(e) (1× 4 marks)
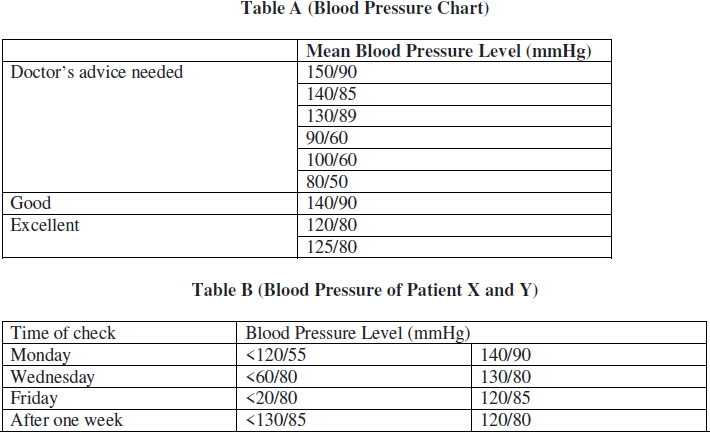
(a) Refer to table B showing the blood pressure of patients X and Y the disease which can be diagnosed from the given data may be
(i) Hypertension
(ii) diabetes
(iii) arthritis
(iv) thyroid
Answer. (i) hypertension
(b) Identify the factors responsible for the disease
Answer. Obesity, lack of physical activity, smoking, more alcohol consumption, stress, etc.
(c) Which one of the following measures would you recommend to the affected patient?
(i) complete bed rest
(ii) high carbohydrate and fat rich diet
(iii) high salt intake
(iv) fat free healthier diet and regular exercise
Answer. (iv) fat free healthier diet and regular exercise
(d) Refer to the table A and suggest the value of the mean blood pressure level beyond which doctor’s advice is sought:
(i) 300/80 mmHg
(ii) 140/80 mmHg
(iii) 130/90 mm Hg
(iv) 150/90 mmHg
Answer. (iii) 130/90 mm Hg
(e) How are the disease like COVID19 most commonly spread?
(i) breathing viruses in air
(ii) hand-to-face contact
(iii) drinking infected water
(iv) Eating contaminated food
Answer. (ii) hand-to-face contact
Question 20. Read the following table and answer any four question from 20(a) to 20(e) (1× 4 marks)
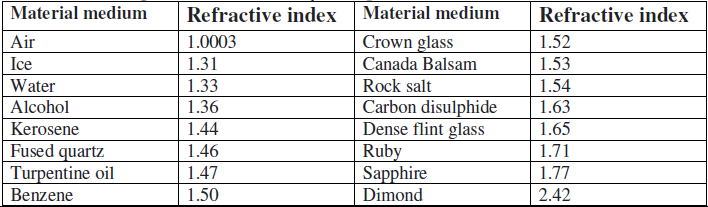
(a) Refractive index is minimum and maximum for
(i) Air and sapphire
(ii) ice and quartz
(iii) vacuum and Diamond
(iv) air and Diamond
Answer. (iii) vacuum and Diamond
(b) the refractive index of crown glass is 1.52 what does it mean ?
Answer. It means that light travels in air 1.52 times faster than it does in crown glass.
(c) What do you infer about the speed of light from the above table ?
(i) Lower the refractive index more the speed of light
(ii) Higher the refractive index more the speed of light
(iii) Higher the refractive index lesser the speed of light
(iv) Lower the refractive index lesser the speed of light
Answer. (iii) Higher the refractive index lesser the speed of light
(d) The material which highest optical density is
(i) Rock salt
(ii) dense glass
(iii) Diamond
(iv) Ruby
Answer. (iii) Diamond
(e) Relative refractive index of ruby w.r.t alcohol is
(i) 1.71
(ii) 1.36
(iii) 1.36/1.71
(iv) 1.71/ 1.36
Answer. (iv) 1.71/ 1.36
SECTION – B
Question 21. What is role of mucus secreted by the gastric glands? (2 marks)
OR
How are the lungs designed in human beings to maximize the area for gas exchange?
Answer. Mucus protect inner lining layer of stomach against HCL fi mucus is not secreted lining of stomach will be destroyed leading acidity and ulcers
OR
A pair of lungs are designed in humans in such a way that they are lined by a thin membrane where the smaller tubes called bronchioles a balloon-like structure and the surface area for the exchange of gases have been increased by the alveoli and network of blood capillaries.
Question 22. Describe the food making process in green plants (2 marks)
Answer. Photosynthesis is the process in which green plants use sunlight to make their own food. Photosynthesis requires sunlight, chlorophyll, water, and carbon dioxide gas. Chlorophyll is a substance in all green plants,
Question 23. What is the unique property of carbon atom? How is this property helpful to us? (2 marks)
OR
Explain why carbon generally forms compounds by covalent bonds
Answer. Catenation and tetravalency, this property help carbon atoms to create large number of compounds
OR
Carbon has 4 electrons in its valence shell. It cannot lose 4 electrons as it involves a lot of energy. Also, it cannot gain 4 electrons because the nucleus cannot hold on to the four extra electrons added. Therefore, to complete the octet, it shares 4 electrons with other atoms. That is why, carbon forms compounds mainly by covalent bonding.
Question 24. Describe the displacement reaction with example. (2 marks)
Answer. Displacement reaction is a chemical reaction in which a more reactive metals displaces a less reactive metals from its solution . any one suitable example
Question 25. Show the direction of light after reflection in given mirror (2 marks)

Answer.

Question 26. Will current flow more easily through a thick wire or a thin wire of the same material when connected to the same source. Why? (2 marks)
Answer. The current will flow more easily through the thick wire than the thin wire. It is because the resistance of a conductor is inversely proportional to its area of cross-section.
SECTION – C
Question 27. Solid calcium oxide was taken in a container and water was added slowly to it. (3 marks)
(a) State two observation made in the experiment.
(b) Write the balanced chemical equation of this reaction.
Answer. (a) Temperature is raised due to the vigorous reaction.
(b) It is an exothermic reaction, heat is observed.
(ii) quicklimes(s)+waterH2O(l)⟶Calcium Hydroxide Ca (OH)2+Δ(heat)
∴ Calcium Hydroxide is produced.
Question 28. Identify the acid and base from which sodium chloride is obtained. Which type of salt it is? When it is called rock salt? (3 marks)
Answer. Acid – Hydrochloric acid (HCl) “”Base – Sodium Hydroxide (NaOH)
Sodium chloride is a neutral salt as pH of its aqueous solution is 7.
Deposits of salt are large crystals that are often brown due to impurities. This is called rock salt.
Question 29. State the function of renal artery, kidney, and urinary bladder. (3 marks)
Answer. Renal artery-Brings impurified blood to kidney for further filtration.
Kidney-Purification and osmo-regulation of blood.
Urinary bladder-Stores urine and allowing urination to be infrequent and controlled.
Question 30. Explain Mendel’s concept of heredity by giving three points (3 marks)
OR
In human beings, the statistical probability of getting either a male or a female child is 50:50. Which of the following is the cause behind it?
Answer. 1. Law of Dominance- dominant alleles always mask the recessive alleles
2. Law of Segregation- during gamete formation the segregation of each gene pair is independent of other pairs
3. Law of Independent Assortment- The Principle of Independent Assortment describes how different genes independently separate from one another when reproductive cells develop.
OR
The sex of an infant is determined by the type of sex chromosome contributed by the male gamete. A male produces two types of sperms, one type bears 22+X composition and the other, 22+Y. Therefore, a male has 50% sperms with X chromosomes and the other 50% with Y chromosomes.
Anyone of the two types of the sperms can fertilize the egg. If a Y bearing sperm fertilizes the egg, the zygote will be a male (XY) and when X bearing sperm fertilizes the egg, the resulting zygote will be female (XX). Since the ratio of the X chromosome and the Y chromosome in a male gamete is 50:50. The statistical probability of male or female infants is also 50:50.
Question 31. You are given three Mirrors of equal size concave, convex and plane. How will you identify them without touching their surfaces? (3 marks)
Answer. – A plane mirror will produce an image of the same size as your face.
– A concave mirror will produce a magnified image of your face.
– A convex mirror will produce a diminished image of your face.
Question 32. A narrow beam PQ of white light is passing through a glass prism ABC as shown in the diagram. (3 marks)
(i) Write the name and cause of the phenomenon observed.
(ii)Where else in nature is this phenomenon observed?
(iii)Based on this observation, state the conclusion which can be drawn about the constituents of white light.
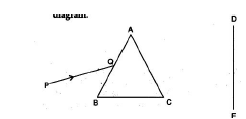
Answer. The diagram is as :
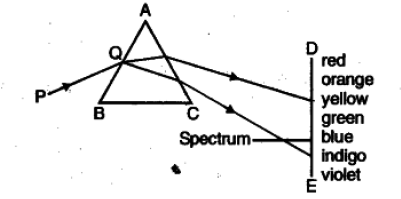
(i) The phenomenon is called dispersion of light. It is because of the reason that different wavelengths of light travel with different speed in the glass prism.
(ii) In a rainbow.
(iii) White light consists of seven different wavelengths viz. violet, indigo, blue, green, yellow, orange and red.
Question 33. Out of the elements H (1), Be (4), Na (11), Mg (12): (3 marks)
(a) Write the pair of elements having similar chemical properties.
(b) State the group number of each pair.
(c) Name one another element belonging to each of these groups.
Answer. a) sodium and magnesium According to the modern periodic law, properties of elements are periodic function of their properties. As Mg is placed right after Na, they have similar properties.
b) hydrogen – group 1 (alkali metals)
Beryllium – group 2 (alkaline earths)
Sodium – group 1
Magnesium – group 2
c) group 1 – lithium
Group 2 – potassium
SECTION -D
Question 34. A non-metal A which is the largest constituent of air, when heated with H2 in 1 :3 ratio in the presence of catalyst (Fe) gives a gas B. On heating with O2 it gives an oxide C. If this oxide is passed into water in the presence of air it gives an acid D which acts as a strong oxidizing agent (a) Identify A, B, C and D (b) To which group of the periodic table does this non-metal belong? (5 marks)
OR
(a) Give reasons for the following:
(i) Zinc oxide is considered as an amphoteric oxide.
(ii) Non-metals in general do not displace hydrogen from dilute acids.
(iii)Metals conduct electricity.
(b) Why do ionic compounds have high melting points?
Answer. (a) A is nitrogen; because nitrogen is the largest constituent of air. B is ammonia, C is nitrogen dioxide and D is nitric acid (nitric acid is a strong oxidizing agent).
When nitrogen is heated with hydrogen in the presence of a catalyst, following reaction takes place:
N2+3H2→2NH3
When nitrogen is heated with oxygen, we get nitrogen dioxide
N2+2O2→2NO2
When nitrogen dioxide is treated with water, we get nitric acid
NO2+H2O→HNO3
(b) The non-metal belongs to group 15 or VA because N has 5 valence electrons (electronic configuration is 2, 5).
OR
(a) (i) Reasons: Zinc oxide can react with both acid and bases, that’s why it is considered as amphoteric oxide.
(ii) Only more reactive compound can displace less reactive compound. Since, non-metals are less reactive than hydrogen. So, they can’t displace hydrogen from diluted acids.
(iii) Metals have free electron through which electricity can flow easily. That’s why metal conducts electricity.
(b) The ionic compounds have strong force of attraction between the oppositely charge ions. To break this force of attraction a lot of heat energy is required. Due to this, ionic compounds have high melting points.
Question 35. Answer the following: (5 marks)
i. How is zygote formed?
ii. State the function of placenta in the mother’s body.
iii. At what interval the egg is formed in human female ovary?
iv. Name two STDs caused by bacterial infection.
v. Why is prenatal sex determination prohibited?
Answer. i. Zygote is formed by the fusion of male and female gamete.
ii. Placenta is a special tissue through which the developing embryo/foetus gets nutrition from mother’s blood. It also transports wastes of the embryo into mother’s blood.
iii. Ovulation releases mature ovum from the ovary. It happens once during a menstrual cycle that is for roughly 28 days.
iv. STDs caused by bacterial infection are Gonorrhea and Syphilis.
v. Prenatal sex determination is misused and it may be the reason for female foeticide.
Question 36. (a) What is an electromagnet? List any two uses. (5 marks)
(b) Draw a labelled diagram to show how an electromagnet is made.
(c) State the purpose of soft iron core used in making an electromagnet.
(d) List two ways of increasing the strength of an electromagnet if the material of electromagnet is fixed
OR
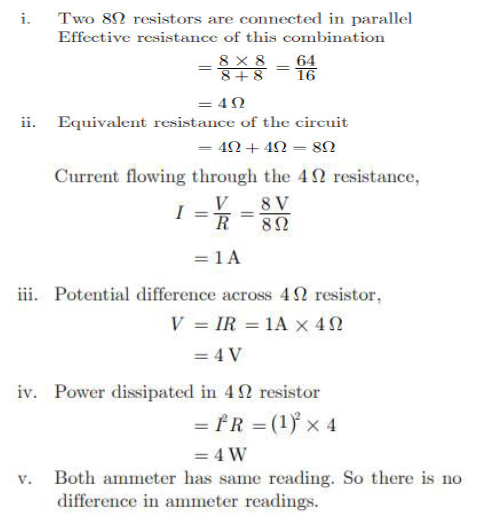
Find out the following in the electric circuit given in Figure.
i. Effective resistance of two 8Ω resistors in the combination.
ii. Current flowing through 4Ω resistor.
iii. Potential difference across 4Ω resistor.
iv. Power dissipated in 4Ω resistor.
v. Difference in ammeter readings, if any.

Answer. (a) An electromagnet is a solenoid with a soft iron core

Uses – 1. Electromagnet are used in all kinds of electric devices like hard disk drive motors, speakers, toys, etc.
2. they are also used in medical equipment like MRI machine used to look organs and structures in our body
(b) in order to make an electromagnet, we take a soft iron rod and wind a coil of insulated copper wire around it. We use a battery to pass a current through the coil. the soft iron rod becomes a complete magnet as long as current id there.
(c) a soft iron core easily gains magnetism when electric current passed through it. It also loses its magnetism as soon current flowing through it stops.
(d) The strength of an electromagnet can be increased by (any two)
1. increasing the number of turns in coil
2. by using soft iron
3. by increasing current
OR
We hoped you liked the Class 10 Science Sample Paper Set C given above. You can refer to more Class 10 science sample papers here. You should also refer to last year paper class 10 science