Please refer to Class 10 Science Sample Paper Set D below provided with solutions. All Class 10 Science Sample Paper have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS. All questions have been provided with solutions.
Class 10 Science Sample Paper Set D
Time: 3 Hours Maximum Marks: 80
General Instructions:
(i) The question paper comprises four sections A, B, C and D. There are 36 questions in the question papers. All questions are compulsory.
(ii) Section–A – question no. 1 to 20 – all questions and parts thereof are of one mark each. These questions contain multiple choice questions (MCQs), very short answer questions and assertion – reason type questions. Answers to these should be given in one word or one sentence.
(iii) Section–B – question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should in the range of 30 to 50 words.
(iv) Section–C – question no. 27 to 33 are short answer type questions, carrying 3 marks each. Answers to these questions should in the range of 50 to 80 words.
(v) Section–D – question no. – 34 to 36 are long answer type questions carrying 5 marks each. Answer to these questions should be in the range of 80 to 120 words.
(vi) There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
(vii) Wherever necessary, neat and properly labeled diagrams should be drawn
SECTION-A
Question 1. What are allotropes? Give one example. (1 Marks)
Answer. Existence of an element in two or more different forms in the same physical state called allotropes. E.g. – graphite Dimond (any one) are allotropes of carbon
Question 2. Define exothermic reaction with example. (1 Marks)
OR
Write the molecular formula and electron dot formula of methane
Answer. Reaction in which heat is evolved called exothermic reaction. Anyone example.
OR
CH4
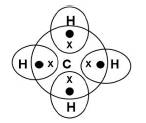
Question 3. What kind of salt is sodium carbonate and why? (1 Marks)
(a) Acidic
(b) Basic
(c) Neutral
(d) Amphoteric
Answer. (b) Basic because it is a slat of weak acid (H2CO3) and strong base (NaOH)
Question 4. On which factors does the electrical resistivity of a given metallic wire depend? (1 Marks)
Answer. It depends on the nature of its material
Question 5. What is unit of electric power? (1 Marks)
Answer. Unit of electric power are joule/second or watt. It can also be expressed in volt ampere.
Question 6. What kind of magnetic field is found near a long straight wire? (1 Marks)
OR
What happens to the current at the time of short circuit?
Answer. The magnetic field near a long wire consists of concentric circles centered on the wire.
OR
The current in the circuit increases heavily.
Question 7. What is a prism? (1 Marks)
Answer. A prizm is an optical device with two triangular bases along with three rectangular lateral surfaces commonly inclined at an angle of 60o.
Question 8. Explain why we see the sign AMBULANCE front of the some vehicles (1 Marks)
Answer. The word AMBULANCE is written inverted because if it is seen through a mirror in the vehicles ahead the drivers see it as AMBULANCE without inversion and hence give way ambulance without any delay.
Question 9. On what factor the colour of the scattered light depends? (1 Marks)
OR
What is dispersion of light?
Answer. Colour of scattered light depends on the size of scattering particles
OR
The phenomenon of splitting of white light into its constituent seven colours on passing through a glass prism is called dispersion of light
Question 10. Define cellular respiration in organisms. (1 Marks)
Answer. Cellular respiration is the process of breaking down of glucose into the energy
Question 11. Explain why, plants have low energy needs as compared to animals (1 Marks)
OR
What is the function of cartilaginous rings found on trachea?
Answer. Plants do not need to move from one place to another. Movements in a plant are usually at the cellular level and hence a far less amount of energy is required by plants.
OR
The C-shaped cartilaginous ring prevents collapsing of trachea, It helps trachea to expand when person is breathing
Question 12. Which energy transformation takes place through autotrophs? (1 Marks)
OR
What does an ecosystem include?
Answer. Autotrophs convert Solar energy into chemical energy by photosynthesis
OR
An ecosystem is an interaction between the living organism (biotic component) and their physical environment (abiotic component).
Question 13. How does nutrition in fungus differ from that in a tapeworm? (1 Marks)
For question numbers 14, 15 and 16, two statements are given- one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both A and R are true, and R is correct explanation of the assertion.
(b) Both A and R are true, but R is not the correct explanation of the assertion.
(c) A is true, but R is false.
(d) A is false, but R is true.
Answer. Because fungus belong to saprophytic mode of nutrition in this nutrition he takes the food
dead and decaying matter. while tapeworm is parasitic nutrition it is depend on host for there food.
Question 14. Assertion (A) :Mineral acids are stronger acids than carboxylic acids (1 Marks)
Reason (R): mineral acid are completely ionized while carboxylic acid ionize partially
Answer. (a) Both A and R are true, and R is correct explanation of the assertion.
Question 15. Assertion (A) :The resistance of an ammeter should be zero (1 Marks)
Reason (R):Ammeter is connected in parallel in a circuit
OR
Assertion (A) :Fleming’s right hand rule gives direction of induced current
Reason (R):Current is induced when a conductor is placed in moving/changing magnetic field
Answer. (c) A is true, but R is false.
OR
(b) Both A and R are true, but R is not the correct explanation of the assertion.
Question 16. Assertion (A) :a male reproductive system the testis are located inside the abdominal cavity (1 Marks)
Reason (R):sperm formation required a slightly lower temperature than the normal body temperature
Answer. (a) Both A and R are true, and R is correct explanation of the assertion.
| Answer Q. No 17 – 20 contain five sub-parts each. You are expected to answer any four sub-parts in these questions. |
Question 17. Read the following passage and answer any four question from 17(a) to 17(e). (1× 4 marks)
Ultraviolet radiation could destroy the organic matter. plant and planktons cannot be thrive, both act as food forland and sea animal, respectively.For humans, excessive exposure to ultraviolet radiation leads to higher risk of cancer (especially skin cancer)and cataracts. it is calculated that every 1 percent decrease in Ozone Layer result in a 2-5% increase in the occurrence of skin cancer.Other ill-effect of reduction of protective Ozone Layer includeincrease in the incidence of cataracts, sunburns and suppression of the immune system.
(a) How is the ozone formed in the atmosphere?
Answer. In atmosphere, some OXYGEN (O2) absorbed energy from ultraviolet (UV) ray and split to for single oxygen atoms. This oxygen combined with remaining oxygen and form ozone (O3) molecule.
(b) What damages the ozone layer?
(i) Chlorofluorocarbons
(ii) Nitric oxide
(iii) free radicals of chlorine
(iv) all of them
Answer. (iv) all of them
(c) Which of the following is global step that has been taken by the world to reduce ozone depletion?
(i) Kyoto protocol
(ii) Paris protocol
(iii)Montreal protocol
(iv) Rio protocol
Answer. (iii) Montreal protocol
(d) In which layer of the atmosphere Ozone Layer is depleting?
(i) Ionosphere
(ii) Stratosphere
(iii) Lithosphere
(iv) Thermosphere
Answer. (ii) Stratosphere
(e) Which type of disease mostly caused by ultraviolet rays.
(i) Diabetes
(ii) Polio
(iii) Heart attack
(iv) Skin cancer
Answer. (iv) Skin cancer
Question 18. Read the following table and answer any four question from 18(a) to 18(e). (1× 4 marks)

(a) what happens to the atomic size as one moves down the group?
(i) Increases
(ii) Decreases
(iii) Remains Constant
(iv) Nothing can be said
Answer. (i) Increases
(b) The element having largest atoms is
(i) Sodium
(ii) Rubidium
(iii) Potassium
(iv) Cesium
Answer. (iv) Cesium
(c) Group – 1 elements are also called
(i) Nobel gases
(ii) Actinides
(iii) alkaline earth metals
(iv) Halogens
Answer. (iii) alkaline earth metals
Question 19. Read the following table and answer any four question from 19(a) to 19(e). (1× 4 marks)
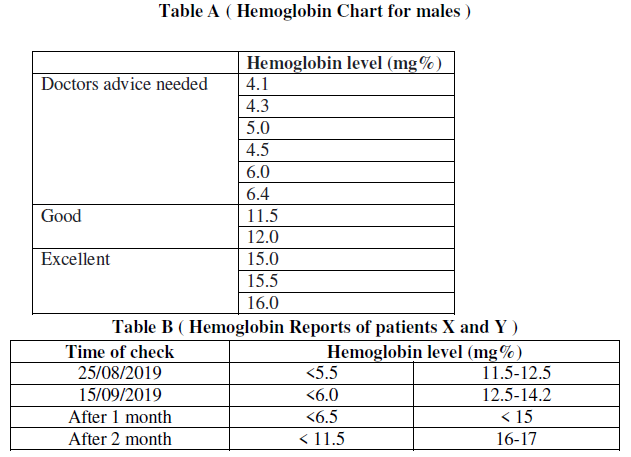
(a) Refer to table B showing the hemoglobin level in blood sample of patients X and Y, the disease which can be inferred from the given data is
(i) Hypertension
(ii) Anemia
(iii) Diabetes
(iv) Leukemia
Answer. (ii) Anemia
(b) The deficiency of which element is responsible for the disease?
(i) Calcium
(ii) Phosphorus
(iii) Silicon
(iv) Iron
Answer. (iv) Iron
(c) Which one of the following diets would be recommended for the affected patient?
(i) Fresh fruits and beans
(ii) Leafy green vegetables and seafood
(iii) Nuts and seeds
(iv) all of them
Answer. (ii) Leafy green vegetables and seafood
(d) Refer to the table A and suggest the value of the mean hemoglobin level beyond which doctorsadvice is sought:
(i) < 5.7
(ii) < 14.3
(iii) > 13.5
(iv) >15
Answer. (i) < 5.7
(e) Which of the following is the most essential nutrient for a woman during her initial stages of pregnancy to prevent birth defects?
(i) Thiamin
(ii) Folic acid
(iii) Vitamin C
(iv) Vitamin A
Answer. (ii) Folic acid
Question 20. Read the following passage and answer any four question from 20(a) to 20(e). (1× 4 marks)
Refraction is the change in direction of light when it passes from the one medium to another. The working of a lens is based on the refraction of light when it passes through one medium to another.A lens is transparent glass which is bounded by two spherical surfaces.The light ray are reflected after passing through the lens.Lenses are two types, convex and concave.
(a) Which physical quantity does not change when light travels from one medium to another?
(i) Speed
(ii) Wavelength
(iii) density
(iv) Frequency
Answer. (iv) Frequency
(b) What do you understand by absolute refractive Index of a medium?
(i) Refractive index of a medium w.r.t air
(ii) Refractive index of a medium w.r.t glass
(iii) Refractive index of a medium w.r.t light
(iv) Refractive index of a medium w.r.t vacuum
Answer. (iv) Refractive index of a medium w.r.t vacuum
(c) How many focal points does a lens have?
(i) One
(ii) Two
(iii) Three
(iv) Four
Answer. (ii) Two (because it has two refracting surface)
(d) Sumeet held a lens at some distance above a paper in such a way that sharp image of the sun was formed on it.In a while, he noticed that there was a hole on the paper where the image was formed. Which type of the lens was it and why did it happen?
(i) Convex
(ii) Concave
(iii) plano-convex
(iv) Plane
Answer. (i) Convex
(e) What is the magnification produced by a plane mirror in what does it mean?
(i) Zero
(ii) + 1
(iii) Between 0 and ∞
(iv) ─ 1
Answer. (ii) + 1 (it means the size of the image is equal to the size of the object)
SECTION – B
Question 21. What is the function of digestive enzymes? (2 marks)
OR
What advantage over an aquatic organism does a terrestrial organism have with regard to obtaining oxygen for respiration?
Answer. Digestive enzymes such as amylase, lipase, pepsin, trypsin, etc. help in the breaking down of complex food particles into simple ones. These simple particles can be easily absorbed by the blood and thus transported to all the cells of the body.
OR
The amount of oxygen present in the water is less in amount compared to the amount of oxygen present in the air. Thus, terrestrial organisms have a advantage over aquatic organisms.
Question 22. Where do plants get each of the raw materials required for photosynthesis? (2 marks)
Answer. The following raw materials are required for photosynthesis:
CO2 enters from the atmosphere through stomata.
Water is absorbed from the soil by the plant roots.
Sunlight, an important component to manufacture food, is absorbed by the chlorophyll and other green parts of the plants.
Question 23. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used? (2 marks)
OR
What is a homologous series? Give example.
Answer. When ethyne is burnt in air incomplete burning takes place and produce sooty flame due to limited supply oxygen present in the air. Which is not enough to melt metals for welding.
OR
A series of carbon compounds having different numbers of carbon atoms but have the same functional group substituting the hydrogen atom is known as a homologous series.
For example, methane, ethane, propane, butane, etc. constitute the alkane homologous series.
The general formula for alkanes is CnH2n+2
Methane CH4; Ethane C2H6; Propane C3H8 and so on.
Question 24. Give reasons why copper is used to make hot water tanks and not steel (any alloy of iron). (2 marks)
Answer. This is because steel contains iron which reacts with steam and form iron oxide. Whereas copper doesn’t react with cold water, hot water and steam. Copper is better conductor of heat than steel.
Question 25. (a) What is visible spectrum? (2 marks)
(b) Why is Red colour used as the stopping light at traffic signal?
Answer. (a) The portion of the electromagnetic spectrum that is visible to the human eye is known as the visible light spectrum.
(b) Red light has highest wavelength of all lights and it can be seen from further distance because it is scattered least by air the effect of scattering is inversely proportional to the forth power of wavelength
Red can easily travel more distance in fog rain and other weather conditions
Question 26. What is (a) the highest, (b) the lowest total resistance that can be secured by combinations of four coils of resistance 4 Ω , 8 Ω , 12 Ω , 24 Ω? (2 marks)
Answer. (a)Highest will be by series =4+12+8+24=48 Ω
(b)Lowest will be by parallel =1/R=1/4+1/8+1/24+1/12=12/24=1/2, so R=2
SECTION – C
Question 27. (a)in electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected (3 marks)
(b) What is observed when a solution of potassium iodide is added to a solution of lead nitrate taken in a test tube? What type of reaction is this?
Answer. (a) In electrolysis, water is decomposed in the presence of electricity to its components. The reaction is shown as below:
2H2O(l)→2H2(g)+O2(g)
As you can see that water splits in to 2 molecules of hydrogen and 1 molecule of oxygen. Since, number of molecules of hydrogen released is double the number of molecules of oxygen released, Volume occupied by hydrogen gas is double the volume occupied by oxygen gas.
(b) When potassium iodide is added to the aqueous solution of lead nitrate then a yellow colored precipitate of Lead iodide is observed. This reaction is a double displacement reaction.
2KI + Pb (NO3)2 —–> PbI2 + 2KNO3.
Question 28. Give reason for the following: (3 marks)
(a) Aluminium oxide is considered as an amphoteric oxide.
(b) ionic compounds conduct electricity in molten state
Answer. (a) Aluminum oxide reacts with both acidic and basic substances to give neutralization reaction and hence cannot be called a true acid as well as base. Hence it is called amphoteric oxide.
Al2O3+6HCl→2AlCl3+3H2O>> Neutralization Reaction with hydrochloric acid (behaves like a base)
Al2O3+2NaOH→2NaAlO2+H2O>> Neutralization reaction with Sodium hydroxide (behave like an acid)
(b) Ionic compounds are bound to each other with strong attraction force. Hence, they are in solid form and their ions are not mobile. When in molten state the ions become mobile and act as carriers for charge and hence conduct electricity.
Question 29. A blue colour flower plant denoted by BB is crossbred with a white colour flower plant denoted by bb. (3 marks)
(a) State the colour of flower we would expectintheirF1 progeny.
(b)Write the percentage of plants bearing white flower inF2generation when the flowers of F1 plants were self pollinated .
(c) State the expected ratio of the genotype BB and Bb inthe F2 progeny.
OR
Give three differences between acquired and inherited traits.
Answer.

F1 Generation
Note: All flowers in the F1 generation are blue.
(b) When flowers of F1 plants are self-pollinated, Then

Hence BB: Bb: bb = 1: 2: 1 is ratio.
OR
1) acquired trait cannot be passed to progeny while inherited traits can be.
2) acquired trait do not bring changes in the DNA of germ cell instead bring in somatic cells while genes of inherited traits are present in DNA of germ cell.
3) acquired traits are acquired during the life of individual while inherited traits are inherited from parents
acquired eg; knowledge inherit eg; eye color, hair color
Question 30. Draw the pattern of magnetic field lines of (3 marks)
(i) A current carrying solenoid
(ii)A bar magnet.
And list two distinguishing features between the two fields.
Answer.

List of the distinguishing features between the two fields (any two)
(1) The poles of the bar magnet do not lie exactly at the end of the magnet but are somewhat inside. In a solenoid, poles can be considered to be lying at the edge.
2) The magnetism retains in the bar magnet naturally but in the solenoid, the magnetism is there so long current flows through it.
3) A magnetic field of a bar magnet emanates from throughout the body of the magnet, with more intensity at the poles. While there is no field emanating from the lateral surface of the solenoid.
Question 31. Give reasons for following: (3 marks)
(a)Arteries are thick-walled.
(b)Why veins have valves?
(c)Plants have low energy needs.
Answer. (a) Since the blood emerges from the heart under high pressure, the arteries have thick, elastic walls.
(b) The main function of the valves in veins is to prevent back flow of blood.
(c) Plants do not need to move from one place to another. Movements in a plant are usually at the cellular level and hence a far less amount of energy is required by plants.
Question 32. (a) Why do we see a rainbow in the sky only after rainfall? (3 marks)
(B)Why is the colour of clear sky blue?
C)What do you mean by atmospheric refraction of light?
Answer. (a) After a shower of rain, rain drops are suspended in air and when a ray of light enters this drop, the drop acts as a prism and splits into its component colours as dispersion takes place. Lights of different colours emerge from the rain drops such as red at the top and violet at the bottom. Thus, a rainbow is formed.
(b) The blue color of the sky is due to the scattering of light by small particles of the atmosphere. Blue is scattered more than any other colors because it travels as shorter, small waves. This is why we see a blue sky most of the time
(c) The refraction of light caused by the earth’s atmosphere is called atmospheric refraction. It is caused due to the varying optical densities of different layers of earth’s atmosphere.
Question 33. Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. Amount and concentration taken for both the acids are same. In which test tube will the fizzing occur more vigorously and why? (3 marks)
Answer. Magnesium metal when reacts with an acid gives off hydrogen gas in the reaction. In test tube A fizzing occurs more vigorously because HCl is stronger acid than acetic acid (CH3COOH). Hence, HCl liberates hydrogen gas more vigorously, which causes fizzing more vigorously.
SECTION – D
Question 34. Of the three metals X. Y and Z. X reacts with cold water. Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in order of increasing reactivity. (5 marks)
OR
Give reasons for the following
(a) Zinc liberates hydrogen gas when it reacts with dilute hydrochloric acid.
(b) Why do ionic compounds have high melting and boiling points?
(c) calcium metal after reacting with water starts floating on its surface.
(d) the surface of some metals acquires a dull appearance when exposed to air for a long time.
(e) Sodium is kept immersed under kerosene oil.
Answer.

OR
(a) Zinc reacts with hydrochloric acid to liberate Hydrogen gas as it is more reactive than Hydrogen and hence, displace it
(b) Ionic compounds are formed by the transfer of an electron between two atoms. Thus, the formation of the cation and anion takes place. Due to very strong electrostatic forces of attraction between cations and anions, these possesses very high boiling / melting point.
(c) After calcium reacts with water, hydrogen gas is released in the reaction. This bubble of hydrogen gas stick to the surface of the metal. The buoyancy of the gas bubbles lifts the calcium up. Therefore, calcium floats on water
Ca(s) + 2H2O(l)→ Ca (OH)2 + H2(g)
(d) The surface of some metals acquires a dull appearance when exposed to air for a long time because metals form a thin layer of oxides, carbonates or sulphide on their surface by the slow action of various gases present in air.
(e) Sodium is a highly reactive element. It reacts with oxygen when it comes in contact with air and burns. To prevent this, it is kept immersed in kerosene.
Question 35. (a) Describe asexual reproduction in hydra. (5 marks)
(b) How does reproduction help in providing stability to the population of species?
Answer. (a) Hydra reproduce asexually by budding. A small outgrowth called bud arises on the parent body. The bud grows and develops mouth and ring of tentacles. the buds break off from the parents and develops into new individuals.

(b) Reproduction is the only means to ensure the continuity of a species. By reproduction, organisms produce large number of new individuals out of which several get perished and only some survive. These surviving organisms replace the naturally dying members of the, population. Hence the population as a whole is not affected and remains stable.
Question 36. Three incandescent bulbs of 100 W each are connected in series in an electrical circuit. In another circuit another set of three bulbs of the same wattage are connected in parallel to the same source. (5 marks)
(a) Will the bulb in the two circuits glow with the same brightness? Justify your answer.
(b) Now let one bulb in both the circuits get fused. Will the rest of the bulbs continue to glow in each circuit? Give reason.
OR
What is Joule’s heating effect? How can it be demonstrated experimentally? List its four applications in daily life.
Answer. (a) Let us assume that resistance of each bulb is R
Case (1) in series connection
Current in each bulb =I= V/3R
Case (2) in parallel connection
Net current =I = 3V/R
Current will get equally divided in three bulbs =1/3 (3V/R) =V/R
Thus, current in parallel is thrice of that in series
Power P=I2R
Thus, bulb in parallel combination will glow more brightly
(b) All bulb stops glowing in series combination as the circuit becomes open. the remaining bulbs glow with same brightness in parallel combination.
OR
When an electric current is passed through a high resistance wire it becomes very hot and produce heat this effect is known as heating effect of current or Jules law of heating.
It states that it states that heat H produced by a resistor of resistance R due to current flowing through it for time t is H= I2Rt.
simple experiment to demonstrate heating effect of current is that if we switch on the bulb for a long period of time then it will become hot.
Application of Joules
heating effect: electric heater, geyser, oven, Bulb, toaster Etc..
We hoped you liked the Class 10 Science Sample Paper Set D given above. You can refer to more Class 10 science sample papers here. You should also refer to last year paper class 10 science
