Please refer to Class 10 Science Sample Paper Set B below provided with solutions. All Class 10 Science Sample Paper have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS. All questions have been provided with solutions.
Class 10 Science Sample Paper Set B
Time: 3 Hours Maximum Marks: 80
General Instructions:
(i) The question paper comprises four sections A, B, C and D. There are 36 questions in the question papers. All questions are compulsory.
(ii) Section–A – question no. 1 to 20 – all questions and parts thereof are of one mark each. These questions contain multiple choice questions (MCQs), very short answer questions and assertion – reason type questions. Answers to these should be given in one word or one sentence.
(iii) Section–B – question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should in the range of 30 to 50 words.
(iv) Section–C – question no. 27 to 33 are short answer type questions, carrying 3 marks each. Answers to these questions should in the range of 50 to 80 words.
(v) Section–D – question no. – 34 to 36 are long answer type questions carrying 5 marks each. Answer to these questions should be in the range of 80 to 120 words.
(vi) There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
(vii) Wherever necessary, neat and properly labeled diagrams should be drawn
SECTION-A
Question 1. Identify the type of reaction that occurs when marble chips and zinc granules are added separately to acid taken in two test tubes. (1 Marks)
OR
Name and state the law which is kept in mind while balancing a chemical equation.
Answer. Single Displacement reaction
OR
Law of conservation of mass
Question 2. Name the substance ,which on treatment with chlorine yields bleaching powder. (1 Marks)
Answer. Slaked lime/Ca(OH)2
Question 3. Draw the electron dot structure of ethane molecule (C2H6). (1 Marks)
Answer.

Question 4. What is the colour of the clear sky during day time? Give reason for it. (1 Marks)
Answer. Clear sky appears blue as it is scattered maximum due to shorter wavelength Reason: When sunlight passes through the atmosphere having the molecules of air and other fine particles, whose size is smaller than the wavelength of visible light, these molecules and particles scatter the blue colour more strongly than the other colours.
Question 5. The radius of curvature of a spherical mirror is 20cm.What is its focal length? (1 Marks)
Answer. f=R/2 , f=20cm/10
f=10cm
Question 6. Name a mirror that can give an erect and enlarged image of an object. (1 Marks)
OR
A ray of light travelling in air enters obliquely into water. Does the light ray bend towards the normal or away from the normal? Why?
Answer. Concave mirror
OR
The light bends towards the normal on entry into water because water is optically denser than air.
Question 7. A coil of insulated copper wire is connected to a galvanometer. What would happen if a strong bar magnet is pushed into the coil ? (1 Marks)
Answer. A momentary deflection is observed in the galvanometer.
Question 8. Write the principle of working of an electric motor. (1 Marks)
Answer. A current –carrying conductor when placed in a magnetic field experiences a force.
Question 9. What is the commercial unit of electric energy? Convert it into joules. (1 Marks)
OR
An electric iron of resistance20 Ω takes a current of 5A.Calculate the heat developed in 30 s.
Answer. Kilowatt hour – Commercial unit of electrical energy 1 kWh = 1000 Wh = 1000 J/S x 3600 sec = 3600000 J = 3.6 x106J
OR
H=I2Rt= 5A×5A×20 Ω×30s= 15000J
Question 10. What is common in the mode of nutrition seen in lice, leeches and tapeworms? (1 Marks)
Answer. Parasitic mode of nutrition
Question 11. Name the component of food that is not digested by our body. (1 Marks)
OR
What is meant by translocation in plants?
Answer. Roughage
OR
The transport of soluble products of photosynthesis is called translocation. It occurs in phloem
Question 12. A food chain comprises of Insects, Hawk, Grass, Snake and frog. Which of these organisms will have the highest concentration of non biodegradable chemicals? Name the phenomenon associated with it. (1 Marks)
OR
Name any two artificial ecosystems.
Answer. Hawk, biomagnification
OR
Garden ,crop fields, aquarium (any two)
Question 13. Which pancreatic enzyme is effective in digesting protein? (1 Marks)
For question numbers 14, 15 and 16, two statements are given- one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
a) Both A and R are true, and R is correct explanation of the assertion.
b) Both A and R are true, but R is not the correct explanation of the assertion.
c) A is true, but R is false.
d) A is false, but R is true.
Answer. Trypsin.
Question 14. Assertion (A):A reaction in which a substance is decomposed into two or more simpler products is known as a decomposition reaction. (1 Marks)
Reason (R):The decomposition of a substance is impossible without supplying energy
Answer. b) Both A and R are true, but R is not the correct explanation of the assertion.
Question 15. Assertion (A) :Ozone is formed in upper atmosphere by O2 in presence of UV radiation (1 Marks)
Reason (R): Ozone depletion will lead to UV ray reaching earth which may cause skin cancer
OR
Assertion (A): Aquarium needs regular cleaning. (1 Marks)
Reason (R): There are no microbes to clean water in aquarium, therefore, it needs to be regularly cleaned
Answer. b) Both A and R are true, but R is not the correct explanation of the assertion.
OR
a) Both A and R are true, and R is correct explanation of the assertion.
Question 16. Assertion (A): Both TT and Tt are tall plants (1 Marks)
Reason (R): A single copy of ‘T’ is enough to make the plant tall
Answer. a) Both A and R are true, and R is correct explanation of the assertion.
| Answer Q. No 17 – 20 contain five sub-parts each. You are expected to answer any four sub-parts in these questions. |
Question 17. Read the following and answer any four questions from 17(i) to (v) (1× 4 marks)
All the living organisms requires food. The food gives energy to the organisms for growth and maintenance of their body functions. The components of food necessary for our body are called nutrients. Green plants prepare their own food while humans and animals are directly or indirectly dependent on plants for their food
(i)Which of the following statements about the autotrophs is incorrect?
(a)They synthesize glucose from carbon dioxide and water in the presence of sunlight and chlorophyll.
(b)They store carbohydrates in the form of starch.
(c ) They convert carbon dioxide and water into carbohydrates in the absence of sunlight.
(d)They constitute the first trophic level in food chains
Answer. (c) They convert carbon dioxide and water into carbohydrates in the absence of sunlight.
(ii) Choose the event that does not occur in photosynthesis :
(a)Absorption of light energy by chlorophyll
(b)Reduction of carbon dioxide to carbohydrates.
(c) Oxidation of carbon to carbon dioxide.
(d)Conversion of light energy to chemical energy.
Answer. (c) Oxidation of carbon to carbon dioxide.
(iii) The small pores present of leaf’s surface are called
(a) Stomata
(b) Chlorophyll
(c) Guard cells
(d)None of these
Answer. (a) Stomata
(iv)In which of the following groups of organism’s food material is broken down outside the body and absorbed?
(a)Mushroom, green plants, Amoeba
(b)Yeast, Mushrooms, bread mould
(c)Paramaecium, Amoeba, Cuscuta
(d) Cuscuta, lice, tapeworm
Answer. (b)Yeast, Mushrooms, bread mould
(v)Two organisms are good friends and live together. One provides shelter, water and nutrients while the other prepares and provides food. Such an association of organisms is termed as
(a)Saprophyte
(b)Parasite
(c) Autotroph
(d)Symbiosis
Answer. (d)Symbiosis
Question 18. The physical and chemical properties of an element mainly depend upon its outer -shell electronic configuration. Since the outer electronic configuration changes as we go from left to right in a period, therefore within the same period, elements show a variation, both in their physical and chemical properties. (1× 4 marks)
On the other hand within a group, the elements have the same electronic configuration.
Therefore, there chemical properties are similar but their physical properties show a regular gradation (variation) as we move down the group from top to bottom due to a corresponding increase in the number of filled inner shells.
(i) When we move left to right in a period, valency of the elements:
(a) Remains the same
(b) Increases
(c) Decreases
(d) Increases and then decreases
Answer. (d) Increases and then decreases
(ii)When we move top to bottom in a group, the atomic size of the elements.
(a) Remains the same
(b) Increases
(c) Decreases
(d) Increases and then decreases
Answer. (b) Increases
(iii)When we move left to right in a period the metallic character of elements:
(a) Remains the same
(b) Increase
(c) Decrease
(d) Increases and then decreases
Answer. (c) Decrease
(iv)When we move left to right in a period the effective nuclear charge acting on the valence shell electron:
(a) Increases
(b) Decreases
(c) Remains the same
(d) Increases and then decreases
Answer. (a) Increases
(v) When we move top to bottom in a group, valency of the elements.
(a) Remains the same
(b) Increases
(c) Decreases
(d) Increases and then decreases
Answer. (a) Remains the same
Question 19. Read the following and answer any four questions from 19 (i) to 19 (v) (1× 4 marks)
Aryan, a Class X student, while experimenting with a thin spherical lens referred to the following ray diagram to demonstrate the image formation. He keeps on shifting the object in front of the lens and obtains various images having different characteristics.

(i) the nature of the lens used is
a) Converging
b) Diverging
c) Plane
d) None of the above
Answer. a) Converging
(ii) Aryan correctly calculates its power as
a) -1.5 D
b) +25.0 D
c) -25.0 D
d) +1.5 D
Answer. b) +25.0 D
(iii) He observed a virtual magnified and erect image. What is the position of the object?
a) 8 cm from lens
b) > 8 cm from lens
c) 16 cm from lens
d) < 4cm from lens
Answer. d) < 4cm from lens
(iv) For obtaining inverted images of different sizes on screen he can position the object
a) At 6cm from lens
b) Beyond C1
c) Only (a)
d) Both (a) and (b)
Answer. d) Both (a) and (b)
(v) The image of an object placed at infinity appears at
a) At C2
b) At F2
c) At F1
d) None of the above
Answer. b) At F2
Question 20. Read the following and answer any 4 questions from 20 (i) to 20 (v). (1× 4 marks)
A solenoid is a long helical coil of wire through which a current is run in order to create a magnetic field. The magnetic field of the solenoid is the super position of the fields due to the current through each coil. It is nearly uniform inside the solenoid and close to zero outside and is similar to the field of a bar magnet having a north pole at one end and a south pole at the other depending upon the direction of current flow. The magnetic field produced in the solenoid is dependent on a few factors such as, the current in the coil, number of turns per unit length etc. The following graph is obtained by a researcher while doing an experiment to see the variation of the magnetic field with respect to the current in the solenoid. The unit of magnetic field as given in the graph attached is in milli-Tesla (mT) and the current is given in Ampere.

(i)What type of energy conversion is observed in a linear solenoid?
a. Mechanical to Magnetic
b. Electrical to Magnetic
c. Electrical to Mechanical
d. Magnetic to Mechanical
Answer. c. Electrical to Mechanical
(ii) What will happen if a soft iron bar is placed inside the solenoid?
a. The bar will be electrocuted resulting in short-circuit.
b. The bar will be magnetized as long as there is current in the circuit.
c. The bar will be magnetized permanently.
d. The bar will not be affected by any means.
Answer. b. The bar will be magnetized as long as there is current in the circuit.
(iii) The magnetic field lines produced inside the solenoid are similar to that of
a. A bar magnet
b. Straight current carrying conductor
c. Circular current carrying loop
d. Eelectromagnet of any shape
Answer. a. A bar magnet
(iv) After analyzing the graph a student writes the following statements.
I. The magnetic field produced by the solenoid is inversely proportional to the current.
II. The magnetic field produced by the solenoid is directly proportional to the current.
III. The magnetic field produced by the solenoid is directly proportional to square of the current.
IV. The magnetic field produced by the solenoid is independent of the current. Choose from the following which of the following would be the correct statement(s).
a. Only IV
b. I and III and IV
c. I and II
d. Only II
Answer. d. Only II
(v) From the graph deduce which of the following statements is correct.
a. For a current of 0.8A the magnetic field is 13 mT
b. For larger currents, the magnetic field increases non-linearly.
c. For a current of 0.8A the magnetic field is 1.3 mT
d. There is not enough information to find the magnetic field corresponding to 0.8A current.
Answer. a. For a current of 0.8A the magnetic field is 13 mT
SECTION-B
Question 21. What is the role of mucus secreted in stomach by the gastric glands? (2 marks)
OR
Write the function of the following in the human alimentary canal :
(i) Saliva (ii) HCl in stomach (iii) Bile juice iv) Villi
Answer. Mucus prevents erosion of inner lining of stomach occurring due to acidity /ulcer caused by HCl
OR
i) Saliva – contains salivary amylase, converts starch to sugar
ii) HCl in stomach – medium acidic/kills pathogen (germs)
iii) Bile- emulsifies fats/neutralizes acidic food in the duodenum
iv) Villi – increases surface area for absorption
Question 22. List two differences between arteries and veins. (2 marks)
Answer. Arteries carry blood away from the heart while veins carry blood towards the heart.
Arteries are thick walled while veins are thin walled.
Valves are absent in arteries while valves are present in veins to ensure that blood flows in one direction only. (any other) (any two)
Question 23. Explain why carbon forms covalent bond? Give two reasons for carbon forming a large number of compounds. (2 marks)
OR
Explain the formation of ammonia molecule
Answer. Carbon has electronic configuration 2, 4. It could gain four electrons forming C-4 anion or lose 4 electrons to form C+4 cation. Both are not possible due to energy considerations. Carbon overcomes this problem by sharing electrons and forming covalent compounds.
OR
Formation of NH3 molecule 2
N – 2, 5
H – 1
Three hydrogen atoms each share their 1 electron with nitrogen to form three covalent bonds and make an ammonia molecule (NH3) ammonia molecule.
Question 24. List in tabular form any two chemical properties on the basis of which metals and non-metals are differentiated. (2 marks)
Answer.
| S.no. | Metals | Non-metals |
| 1 | Lose electrons to form positive ions/are electropositive in nature | Gain electrons to form negative ions/ are electronegative in nature |
| 2 | React with dilute acids to liberate hydrogen gas | Do not react with dilute acids |
| 3 | Generally metal oxides are basic in nature | Generally non-metal oxides are acidic in nature |
Question 25. (i) What is visible spectrum? (2 marks)
(ii)Two triangular glass prisms are kept together connected through their rectangular side. A light beam is passed through one side of the combination.
Will there be any dispersion? Justify your answer.
Answer. (i) Visible spectrum is the band of colored components of a white light beam.
(ii) The given setup will behave like a glass slab, resulting in recombination of the seven colors to produce white light.
Question 26. Mention two reasons why tungsten is used for making filaments of electric lamp. (2 marks)
Answer. (i) High resistivity
(ii) High melting point.
SECTION-C
Question 27. With the help of a flow diagram, how would you establish that in human beings the sex of a newborn is purely a matter of chance and none of the parents may be considered responsible for a particular sex of a newborn child? (3 marks)
OR
What are chromosomes? Explain how in sexually reproducing organisms the number of chromosomes in the progeny is maintained.
Answer. (a) Male x Female
XY XX
Gametes: X,Y X
Zygote: XX XY
Girl Boy
Sex determination is purely by chance.
The fusion of a particular sperm with an egg is purely a matter of chance
OR
* Chromosomes are thread like structures present in nucleus containing genetic material / DNA
* Number of chromosomes are reduced to half during gametes / germ cell formation.
* After fertilization of germ cells the number of chromosomes is maintained in progeny
Question 28. (a) Construct a terrestrial food chain comprising four trophic levels. (3 marks)
(b) What will happen if we kill all the organisms in one trophic level?
(c) Calculate the amount of energy available to the organisms at the fourth trophic level if the energy available to the organisms at the second trophic level is 2000 J.
Answer.

Question 29. Describe the structure and function of the basic filtering unit of kidney nephron. (3 marks)
Answer. Structure: Cluster of blood capillaries / glomerulus is associated with cup shaped structure called Bowman’s capsule, which leads to coiled tubular part of Nephron.
Function: Collects the filterate and reabsorbs useful substances like glucose, amino acids, salts and water from filterate and forms urine.
Question 30. A compound ‘A’ is used in the manufacture of cement. When dissolved in water, it evolves a large amount of heat and forms compound ‘B’. (3 marks)
(i) Identify A and B.
(ii) Write chemical equation for the reaction of A with water.
(iii) List two types of reaction in which this reaction may be classified.
Answer. (i) A=CaO / Quick lime/ Calcium oxide
B = Ca(OH)2 / Slaked lime / Calcium hydroxide
(ii) CaO+H2O →Ca(OH)2+ heat or energy
(iii) Combination reaction
Exothermic reaction
Question 31. (a) What was the basis of Mendeleev’s classification of elements? (3 marks)
(b) List two achievements of Mendeleev’s periodic tables.
Answer. a) Atomic mass
b) (i) He could classify all the 63 elements known at that time
(ii) He left gaps for the yet to be discovered elements.
(iii) He predicted the properties of such elements. (any two)
Question 32. What happens when (Write the balanced equation involved) – (3 marks)
(i) Copper is heated in air?
(ii) Aluminum oxide is reacted with hydrochloric acid?
(iii) Potassium reacts with water?
Answer. (i) 2Cu + O2 → 2CuO
(ii) Al2O3 + 6HCI → 2AICI3 + 3H2O
(iii) 2K2H2O → 2KOH + H2
Question 33. What happens after refraction, when : (3 marks)
(i) a ray of light parallel to the principal axis passes through a concave lens?
(ii) a ray of light falls on a convex lens while passing through its principal focus?
(iii) a ray of light passes through the optical centre of a convex lens?
Answer.

SECTION-D
Question 34. (a)What does pH scale measure ? (5 marks)
(b) Write its range.
(c)pH has a great importance in our daily life” explain by giving three examples.
OR
(a)compound which is prepared from gypsum has the property of hardening when mixed with a proper quantity of water. Identify the compound and write its chemical formula. Write the chemical equation for its preparation. Mention any one use of the compound.
(b) Why is electrolysis of brine called ‘Chlor-alkali process’? Write the chemical equation involved in this process.
Answer. (a) a) pH scale measures the hydrogen ion concentration in a solution thus indicating acidic/basic nature of a solution.
b) From 0 to 14
(c)Any three point given above –
1. Plants and animals are pH sensitive. Living organisms can survive only in narrow range of pH change.
2. pH of the soil. Plants require a specific pH range for their healthy growth.
3. pH in our digestive system. Our stomach produces hydrochloric acid that helps in the digestion of food. During in digestion the stomach produces too much acid that cause pain and irritation.
4. Change in pH causes tooth decay. Tooth decay start when the pH of the mouth is lower than 5.5. Tooth enamel gets corroded when the pH in the mouth is below 5.5.
5. Self-defense by plants and animals through chemical warfare. Bee– sting leaves and acid causing pain and irritation. Applying a mild base like baking soda on the stung area provides relief.
OR
(a)The name of the compound is Plaster of Paris
Its chemical formula is CaSO4. ½ H2O
Equation:
CaSO4.2H2O ————-> CaSO4. ½ H2O + 1 ½ H2O
It is used in the hospitals mainly as plaster for supporting fractured bones in the
right position
(b) The products formed are ‘chlor’ for chlorine and ‘alkali’ for sodium hydroxide.
2 NaCl (aq) + 2 H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Question 35. (a) Write the function of following parts in human female reproductive system : (5 marks)
(i) Ovary
(ii) Oviduct
(iii) Uterus
(b) Describe in brief the structure and function of placenta.
Answer. a. i) Ovary – releases egg/ female gamete/ ovum releases estrogen/ female hormones (any one)
ii) Oviduct- Transportation of ovum/ egg from ovary to the uterus/ Site of fertilization
iii) Uterus – Development of embryo/ foetus
b) Placenta- It is a disc embedded in uterine wall which contains villi on the embryo side of the tissue and blood space on mother side.
Function of placenta: Provides nourishment to embryo from mother’s blood / Removal of waste from embryo to mother’s blood. (Any one)
Question 36. (a) List two disadvantages of using a series circuit in homes. (5 marks)
(b) Calculate the effective resistance between A and B in the circuit given below :
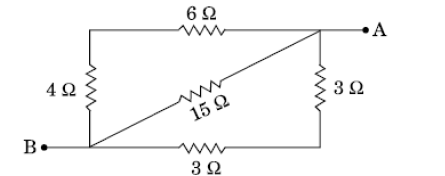
OR
(a) Derive the relation for the equivalent resistance when three resistors of resistances R1, R2 and R3 are connected in parallel.
(b) Find the minimum resistance that can be made using four resistors, each of 20 Ω.
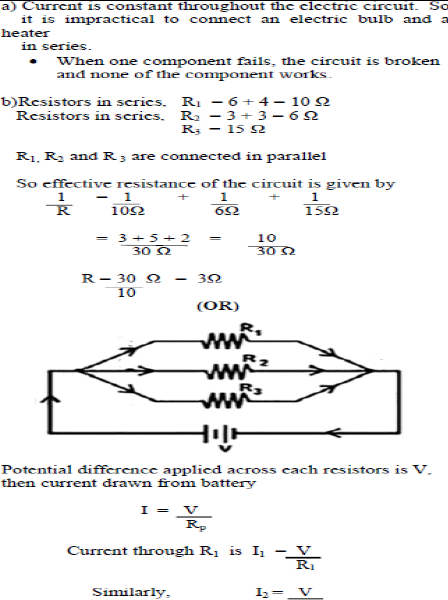
Answer.

We hoped you liked the Class 10 Science Sample Paper Set B given above. You can refer to more Class 10 science sample papers here. You should also refer to last year paper class 10 science